CST 103:Chapter 26: Genitourinary Surgery
1/42
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
43 Terms
What is dialysis and how does it work?
The kidneys normally remove waste products from the blood. Without this function, the body becomes quickly weakened by toxins produced during normal metabolism. Kidney dialysis is a procedure that performs this function in patients with chronic and end-stage renal disease. The two types of kidney dialysis are hemodialysis and peritoneal dialysis. Dialysis is performed regularly, or it can be done as an emergency procedure to remove ingested toxins from the blood that otherwise would lead to immediate kidney failure.
A small incision made in the urethra to reduce scarring or relieve a stricture.
Urethrotomy
A procedure in which ultrasonic sound waves are used to pulverize kidney or gallbladder stones
Extracorporeal shock wave lithotripsy (ESWL)
The removal of tissue or an organ without prior fragmentation or dissection
Enucleation
Removal of all or part of the prepuce (foreskin) of the penis
Circumcision
Inflammation of the glans penis
Balanitis
Condition in which the foreskin cannot be retracted from the glans.
Phimosis
Surgical creation of vascular access for patients undergoing hemodialysis
A-V shunt
Is performed to detect specific substances, both normal and abnormal, in urine.
Urinalysis
The presence or absence of specific substances in the blood reveals kidney function
Blood test
Measures the rate of creatinine clearance from the blood
GFR glomerular filtration rate
Is a test that assesses the elimination of urea from the liver
Blood urea nitrogen
Tissue removed from the genitourinary (GU) tract for microscopic pathological testing.
Tissue biopsy
Is preferred method for imaging tumors of the kidney.
Computed tomography (CT)
C-arm.
Fluoroscopy
This process involves radiograph studies using a contrast media.
IV urography
This is a radiograph of the kidney, ureters, and bladder.
KUB
This study provides images of the bladder while it is emptying.
MCU Micturating cystourethrogram
Provides an extremely detailed assessment and is commonly used in the diagnosis of tumors.
Magnetic resonance imaging (MRI):
This is used in GU studies to detect metastasis arising from a primary tumor of the prostate.
Nuclear imaging
Injections are made using a catheter inserted into the ureter.
Retrograde ureteropyelography:
This is used in the assessment of patients who are ineligible for CT or other forms of radiographic exposure.
Ultrasonography:
Case Study
You are about to scrub for a Cystoscopy when the operating room supervisor delivers a a surgical tech student to you and asks you to serve as her preceptor for the case. Your student has never seen a Cystoscopy before, and you decide to describe the procedure to her before you start. What steps would you describe to her to explain the procedure?
The patient is placed in the supine position on the urology table. Low lithotomy stirrups or knee crutches are used to abduct and externally rotate the patient's legs. 2 The patient is prepped and draped for a perineal procedure. 3 A topical anesthetic or water-soluble anesthetic solution is instilled into the urethra. 4 Urethral dilation is performed as needed. 5 The sheath and telescope or obturator are lubricated and inserted into the urethra. 6 The obturator is removed. 7 The bladder is filled with a distention medium. 8 The surgeon examines the urethra and bladder from all angles. 9 Diagnostic and operative procedures are performed.
During cystoscopic procedures, the bladder is distended with fluid to enhance visualization of the internal structures. Describe the following solutions, which are commonly used in cystoscopic procedures, and explain when they might be used.
A. Sorbitol
B. Glycine
C. Sterile distiller water
D. Water
Labeling (part 2) answers/components of male reproductive system
Explain the purpose of continuous irrigation during transurethral surgery.
Explain how a urethral catheter is used as a tamponade.
What is a French (Fr) size? What is the most common catheter size for an adult?
Explain why testicular torsion is an emergency.
The lateral position is used for many procedures of the genitourinary tract. List at least five critical safety considerations for this position. Include specific anatomical locations and risk factors.
What surgical approaches are used to enter the retroperitoneal cavity?
What specific psychological considerations are important for the male patient undergoing surgery of the external genitalia?
Many patients undergoing transurethral surgery are older. List four methods you would use to communicate with these patients.
Testicular cancer is the most common cancer among young men. How would you approach a 26-year-old patient undergoing surgery for testicular cancer? Be specific. Define the patient's emotional needs at the time of surgery as part of your response.
TURP
Surgical Goal
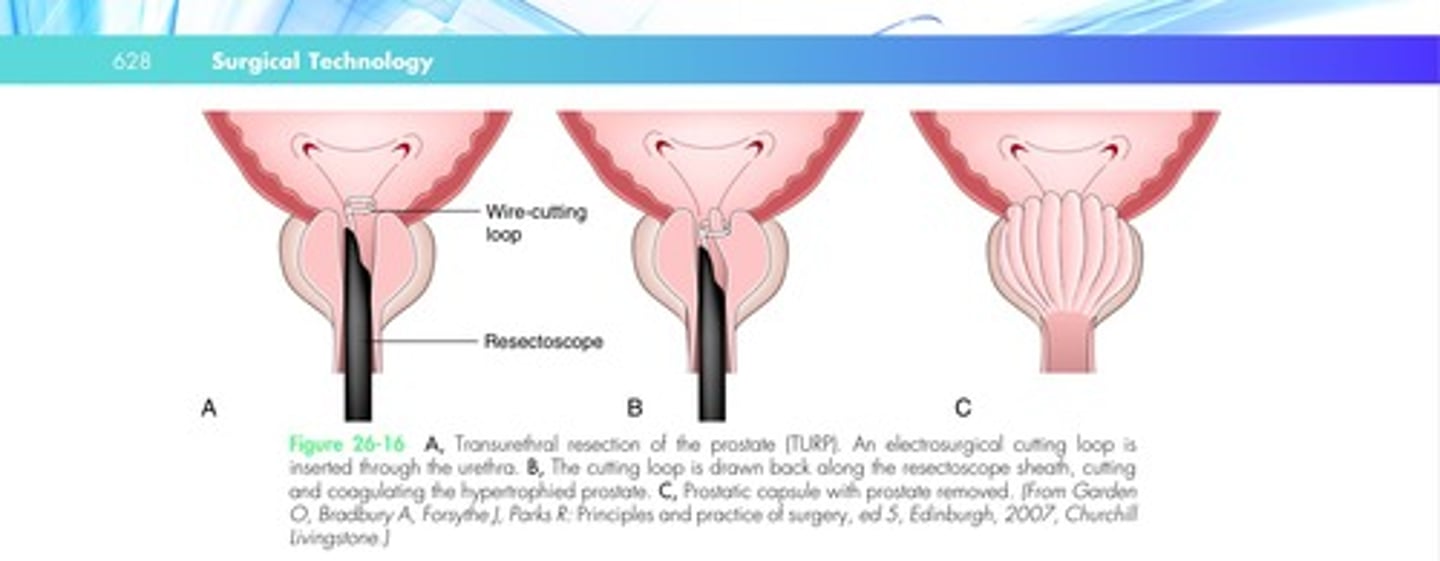
In transurethral resection of the prostate (TURP), the prostate is removed with a resectoscope inserted through the urethra.
Pathology
Enlargement of the prostate generally is related to infection, a benign tumor, or malignancy.
Benign prostatic hyperplasia is nonmalignant enlargement of the prostate gland, which can occur in men older than 40.
The prostate enlarges in two ways. In one type of growth, cells multiply around the urethra, causing obstruction.
In the other type of growth, or middle lobe prostate growth, cells grow into the urethra and the bladder outlet area.
Obstructive disease may cause reflux (backward flow) of urine, infection, and difficulty voiding.
Benign prostatic hyperplasia is commonly treated by resection.
Discussion
The patient is placed in the lithotomy position.
A routine cystoscopy is performed with a 30-degree lens to evaluate the bladder and other structures.
During resection, continuous irrigation or bladder distention with a nonelectrolytic solution (e.g., sorbitol or glycine) is used to maintain a clear surgical field and to evacuate small pieces of tissue.
Continuous irrigation permits clear visualization during resection.
The resectoscope is constructed with an outer sheath that allows fluid to flow out of the instrument.
Irrigation fluid must be maintained.
As mentioned, a solution warmer is used to prevent hypothermia.
The surgeon lubricates the cystoscope and inserts it into the urethra.
The obturator then can be removed, allowing the bladder to drain.
The cysto assistant should be prepared to collect urine from this sample, because it will be submitted for analysis.
Irrigation fluid is then instilled into the bladder. The bladder is assessed, and the cystoscope is removed.
The urethra is then dilated with van Buren sounds.
The resectoscope is inserted, and resection begins at the middle and lateral lobes and continues in a systematic pattern.
The small pieces of tissue that are released into the irrigation fluid may be evacuated with an Ellik evacuator or a Toomey syringe.
The technologist must retain all pieces of specimen for pathological examination in a small basin.
After resection, a three-way Foley catheter is inserted, and the bladder is irrigated to ensure adequate flow and hemostasis.
The catheter remains in place for 12 to 24 hours.
Other minimally invasive techniques used in prostatectomy include laser and transurethral needle ablation using ultrasound.
TURP is illustrated in Figure 26-16.
After a TURP procedure, the patient may remain catheterized for several days to facilitate irrigation of the bladder.
Possible postoperative complications include:
•Incontinence
•Impotence
•Infertility
•Passage of semen into the bladder instead of the urethra (retrograde ejaculation)
•Urethral stricture
TURP diagram
TURB
Note: Transurethral resection of a bladder tumor is performed in the same manner as TURP.
Prostatectomy
Prostatectomy traditionally has been performed by cystoscopic resection or open surgery using various perineal and suprapubic techniques.
Modern prostatectomy is performed laparoscopically, robotically, or through an open approach (perineal or suprapubic) and by cystoscopic resection.
Brachytherapy, which involves the implantation of radon seeds, and cryosurgery also are done to treat prostate cancer.
PERINEAL PROSTATECTOMY
Surgical Goal Perineal prostatectomy is the removal of a prostatic adenoma through a perineal approach.
In the past, prostatectomy often resulted in impotence and incontinence.
Nerve-sparing procedures now are practiced to prevent these complications.
Pathology
Cancer of the prostate is a slow-growing cancer.
There are about 230,000 cases and 40,000 deaths per year in the United States.
A prostate-specific antigen (PSA) blood test and digital rectal examination are the initial tests.
A percutaneous biopsy is performed if the results of these tests are abnormal.
Surgical intervention is discussed with the patient, and all treatment options are provided in counseling.
1. The patient is placed in the high lithotomy position.
2 An inverted U-incision is made in the perineum and carried through the muscle and fascia.
3 The central tendon is isolated and divided.
4 The rectourethral muscles are divided and retracted.
5 Frozen section biopsies are removed from the prostate.
6 The urethra is clamped and divided.
7 The prostatic capsule is incised, and the prostate is removed.
8 The capsule is closed.
9 A Foley catheter is inserted.
10 The urethra is anastomosed for continuity.
11 The wound is closed in layers.
Discussion
A perineal approach to prostatectomy may include laparoscopic removal of lymph nodes during a combined or a separate procedure.
Lymph node analysis is performed for cancer staging.
The patient is placed in the exaggerated lithotomy position with the legs well above the pelvis and the buttocks brought to the edge of the operating table break.
To further lift the sacrum and pelvis, a gel pad is placed under the sacrum.
This is an extreme position that puts strain on the lower back, hips, and sacrum.
Positioning must be performed by trained personnel with attention to risk factors.
The patient is prepped for a perineal incision extending to the abdomen, midthigh, anus, and lower sacrum.
A Foley catheter is placed in the bladder.
Draping exposes the perineum.
An adherent barrier drape with anal pouch is used for the procedure.
The pouch provides an inlet through which the surgeon can digitally support the roof of the rectum during dissection.
To start the procedure, the surgeon passes a Lowsley traction device through the urethra and into the bladder.
This pushes the bladder down toward the perineum.
A U-shaped incision is made in the perineum with the skin knife. Bleeders are controlled with the ESU.
The surgeon then places several Allis clamps on the incision edges for retraction.
The incision is extended to muscle and fascia with the ESU.
The central tendon and rectourethral muscle are isolated and divided.
The levator muscle is retracted to expose the prostatic capsule and prostate gland.
The surgeon may use digital support through the anus to assist dissection.
Small sponge dissectors, Metzenbaum scissors, and the ESU are used to extend the dissection and isolate the prostate.
Important points or planes of dissection are those between the prostate and urethra, bladder neck, anterior bladder, seminal vesicles, and rectum.
Right-angle clamps are needed to isolate the neurovascular pedicles and to divide and ligate with suture, LigaSure, or ligation clips.
Dissection of the vascular system is carefully extended on both sides of the prostate.
In nerve-sparing procedures, no electrosurgical coagulation is used to separate the prostate from the surrounding tissue, which contains the nerves and blood vessels that innervate the penis for erectile function.
The urethra is isolated from the base of the prostate and bladder neck.
It is then double-clamped and divided, preserving the bladder neck.
This leaves a small urethral stump at the bladder neck.
The wound is irrigated, and bleeders are controlled with the ESU and fine suture ligatures.
With dissection completed, the prostate can be enucleated from the capsule.
The urethral stumps are then anastomosed to bypass the prostate.
Before the anastomosis is completed, a Foley catheter is inserted.
The anastomosis is done with fine nylon or some other synthetic monofilament suture.
The closure is tested by instilling saline into the bladder.
When bleeding has been controlled, the wound is irrigated with warm saline.
Drains are placed in the wound and brought out through the incision or a separate stab incision.
Closure is completed in layers with 2-0 and 3-0 absorbable synthetic sutures.
The skin is closed with 3-0 or 4-0 subcuticular or interrupted sutures.
The wound is dressed with gauze fluff squares and an absorbent pad to absorb drainage.
A perineal prostatectomy is shown in Figure 26-27.
An indwelling Foley catheter is left in place for 1 to 2 weeks after the procedure.
This allows the urethral anastomosis to heal.
Recovery from an open procedure takes considerably longer than from laparoscopic or robotic-assisted surgery.
Complications include postoperative bleeding, retrograde ejaculation, failure to ejaculate, and urinary retention.
Suprapubic prostatectomy
SUPRAPUBIC PROSTATECTOMY
Surgical Goal In a suprapubic prostatectomy, the prostate is removed through a suprapubic incision.
Pathology
Prostatectomy is performed to treat benign prostatic hypertrophy and for cancer of the prostate.
If the patient has been diagnosed with cancer, the lymph nodes are removed to stage the disease.
Discussion
The patient is placed in the supine position.
Padded shoulder braces may be required for the Trendelenburg position.
However, these are used only if absolutely necessary because of the increased risk to the brachial plexus, discussed in Chapter 19.
A Foley catheter is inserted before the prep.
The patient is prepped and draped for a suprapubic incision.
The surgeon makes a transverse or longitudinal suprapubic incision into the space of Retzius.
A self-retaining retractor is placed in the wound.
Two traction sutures or Allis clamps are placed in the bladder wall, and an incision is made between them.
The bladder edges are then grasped with Allis clamps and retracted upward.
The scrub should have suction available to drain the bladder.
A Judd or Deaver retractor is placed in the bladder, and the prostatic mucosa is incised with the ESU.
The bladder retractors are then removed.
Using the fingers, the surgeon performs an enucleation of the prostate.
In this technique, the tissue is removed en bloc without trauma to the fossa or bed of the tissue.
The bladder retractors are replaced, and the wound is checked for bleeding.
The fossa may be packed with sponges to secure hemostasis.
Large bleeding vessels are ligated with size 0 or 2-0 absorbable suture ligatures.
Capillary bleeding is controlled with hemostatic agents (e.g., Surgicel, Avitene, or Gelfoam).
A Malecot or Pezzer catheter is placed in the wound and brought out through a small stab incision near the wound edge.
The bladder is then closed in two layers with absorbable sutures.
The wound is irrigated, and a wound drain is placed in the cavity.
The incision then is closed in layers.
Related Procedures
RETROPUBIC PROSTATECTOMY
In a retropubic prostatectomy (Figure 26-28), the surgery is approached from a lower midline incision.
The prostate is dissected from the anatomical attachments discussed previously and enucleated.
The prostatic capsule is closed with sutures, and the urethra is anastomosed to the bladder neck.
In this approach, the margins of the prostate are sent for frozen section, and lymph node removal is performed at the time of prostate surgery.
The results of the frozen section determine the need for wider pelvic excision.
LAPAROSCOPIC PROSTATECTOMY
Laparoscopic prostatectomy is performed with the patient in the low lithotomy position.
Three or four trocar ports are used, including an umbilical site for the camera port.
After a pneumoperitoneum has been established, the camera port is placed and the remaining trocars are inserted under direct vision.
The procedure follows anatomical dissection for the open prostate technique.
The prostate is systematically dissected from the bladder, seminal vesicles, urethra, rectum, and vascular bundles.
The ESU or LigaSure system is used to coagulate and to cut away the margins of the prostate, which contain the neurovascular supply necessary for erectile function.
Endoscopic clips are used to ligate the two vascular bundles on either side of the prostate.
Once the dissection planes have been established and the prostate can be enucleated from the capsule, the urethra is divided as in an open procedure.
The prostate is removed through an endoscopic collection sac.
Continuity of the urethra is reestablished after insertion of a Foley catheter.
Anastomosis of the urethra to the bladder is performed in two layers using continuous fine monofilament sutures.
The Foley catheter remains in place.
Bleeding is controlled, and the wound is irrigated with warm saline.
The ports are withdrawn, and the incisions are closed with one or two layers of absorbable synthetic suture and Steri-Strips.
Following laparoscopic or robotic-assisted surgery, the patient can be discharged from the hospital within 1 or 2 days.
The urethral catheter remains in place to allow the urethral anastomosis to heal.
Patients experience some level of incontinence in the recovery period.
Nerve-sparing procedures provide a greater level of continence and erectile function.
ROBOTIC-ASSISTED PROSTATECTOMY
Robotic-assisted laparoscopic prostatectomy follows the surgical techniques used in the routine laparoscopic procedure, with some technical differences.
The robotic arms are used to guide the instruments, and the monitor view is threedimensional and magnified by 10.
Discussion
The preparation of the patient is the same as for laparoscopy.
The patient is placed in the lithotomy position, and the robotic arms enter from the side.
The surgical technologist and the surgeon's assistant operate at the bedside.
A Foley catheter is inserted and manipulated during the surgery to elevate and identify the urethra intraoperatively.
The surgeon initially scrubs in with the sterile team in order to site and place the cannulas that receive the camera and robotic instruments.
She or he then shifts to the robotic console to perform the surgery.
At the start of surgery, the surgical technologist should have instruments available for insertion of cannulas.
The anatomical and surgical steps of the procedure are the same as those described for laparoscopy.
After placement of the optical trocar, the remaining trocars and cannulas are inserted.
The surgeon may use a ruler to measure the distance between the camera and other cannula sites.
Once the trocars are in place, the surgical technologist removes instruments used in the trocar placement from the Mayo and replaces these with robotic instruments.
A 0-degree camera is most commonly used during the procedure.
A major portion of the dissection is performed using a bipolar fenestrated grasper, ESU hook probe, and dissection scissors.
A Hem-o-Lok applier and clips should be available for bleeders.
One of the advantages of the robotic procedure is the reduction of intraoperative bleeding owing to the pneumoperitoneum, which acts as a tamponade on the large veins that normally bleed profusely during open prostatectomy.
Also, robotic surgery allows a much finer dissection and greater control of blood vessels because the robotic instruments are able to enter into much smaller spaces than is possible with open or even MIS surgery alone.
As the dissection continues, the Foley catheter is manipulated by the surgeon's assistant or the surgical technologist as directed by the surgeon to verify the course of the urethra through the prostate.
The prostate is dissected from the bladder, urethra, rectum, seminal vesicles, and vascular bundles.
The dorsal vein is ligated using size 0 Vicryl or Monocryl.
Once the urethra has been dissected from the prostate tissue, it is anastomosed to the bladder neck using 2-0 Monocryl on a double-arm suture.
The bladder is irrigated at this point with a catheter tip syringe to check for any gaps in the anastomosis.
Gelfoam and Surgicel topical hemostats may be used for hemostasis at the anastomosis.
The prostate is removed through one of the trocars using a specimen retrieval bag (see Specimen Retrieval in Chapter 24).
Before removing the trocars, a small drain may be inserted and attached to the skin during closure.
A final count is performed and the robotic instruments withdrawn.
The incisions are closed using size 1 PDS or 0 Vicryl for the fascia and 4-0 Monocryl for the skin.
Dermabond is also applied over the skin sutures, and finally Band-Aid dressings.
The patient retains a Foley catheter in the immediate postoperative period up to 1 week or more.
Most patients are able to be discharged after robotic prostatectomy after 1 to 3 days.
The patient will return for follow-up testing and further treatment as required.
1 The patient is prepped and draped for an abdominal laparoscopic procedure in the lithotomy position.
2 A pneumoperitoneum is established using a Veress needle.
3 An optical trocar is inserted through a small periumbilical incision, and the robotic camera is placed.
4 The remaining trocars are sited and placed.
5 The robot is docked and arms locked into the cannulas.
6 The prostate is dissected from the surrounding structures.
7 The major prostatic vessels are identified and ligated.
8 Dissection is completed.
9 The urethra is reattached to the bladder neck.
Ileal conduit
ILEAL CONDUIT
Surgical Goal
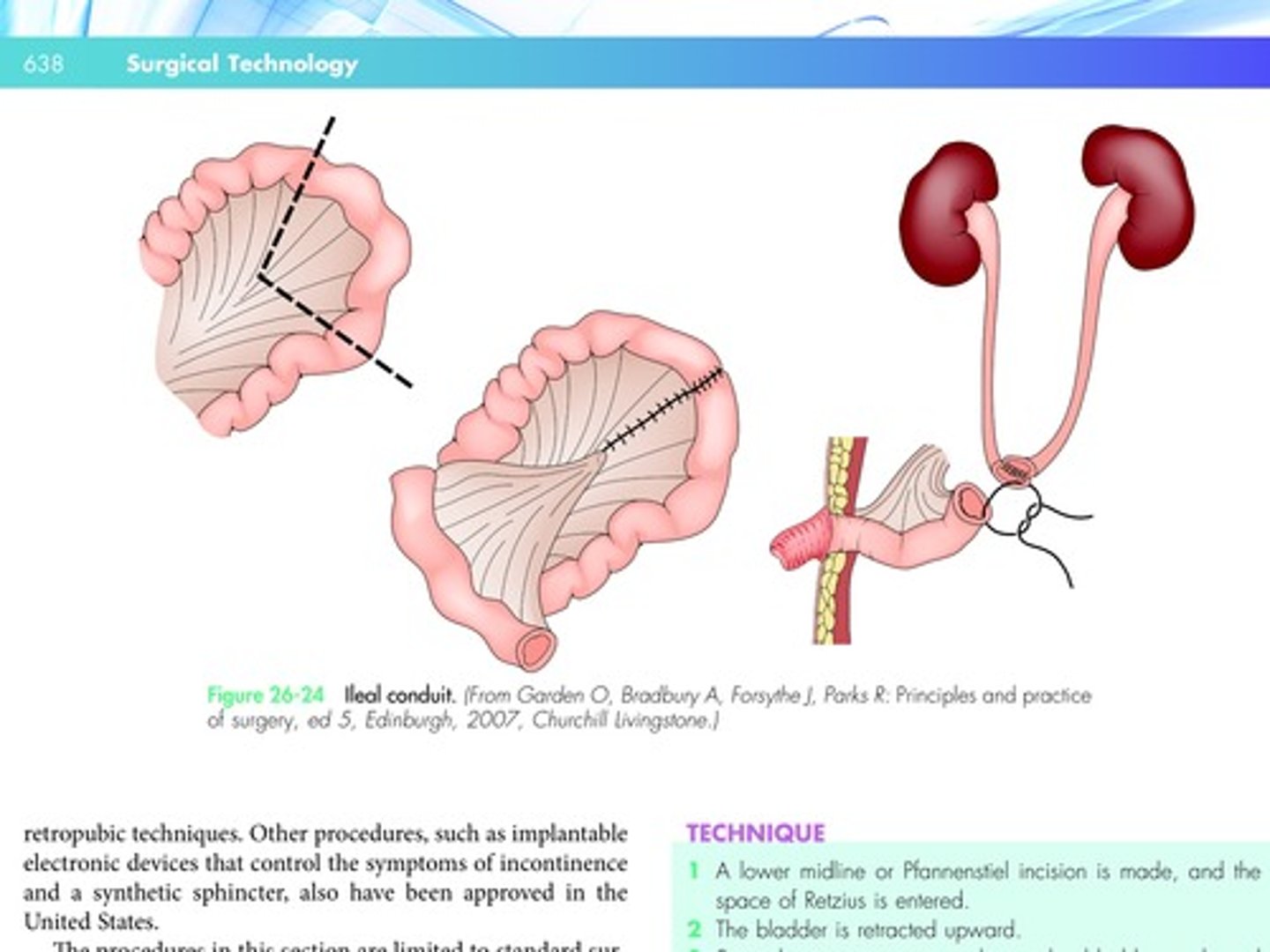
In an ileal conduit procedure, a functional bladder is constructed with a loop of bowel that is brought out of the abdominal wall.
A stoma is created for urine drainage.
This procedure has been widely successful for urinary diversion.
Pathology
Urinary diversion away from the bladder is performed before or after a radical cystectomy, in which the bladder and surrounding tissue
have been removed as a treatment for cancer.
Discussion
Many of the techniques used in this procedure are discussed as part of the bowel procedure (see Chapter 23).
In preparation for the procedure, the scrub should have gastrointestinal and long instruments available.
The patient is placed in the supine position, and a Foley catheter is inserted.
The patient then is prepped and draped for an abdominal incision (usually a low midline incision).
To begin the procedure, the surgeon enters the abdomen and retroperitoneal cavity.
A large self-retaining retractor is placed in the wound.
A portion of the large intestine and adjoining ileum is mobilized, as for a bowel resection.
A linear stapling instrument may be used to resect the proximal limb of the ileum.
The two severed ileal limbs are then anastomosed.
When traditional suturing methods are used, four intestinal clamps are placed across a segment of the ileum, two at each end.
The surgeon then divides the ileum in both locations, cutting between the sets of clamps with the ESU.
The proximal end of the ileum is closed with a double layer of absorbable suture.
The surgeon identifies the ureters and may place a small Penrose drain around them for retraction.
The ureters are divided from the bladder, and an end-to-side anastomosis is made between the ureters and the isolated segment of the ileum.
The anastomosis is performed with 4-0 interrupted sutures of absorbable material.
Stoma
Formation To perform the ileostomy, the surgeon first incises the skin over the area of the proposed stoma, excising a small disc of tissue from the abdominal wall.
Dissection is taken down to the rectus muscle.
A Kelly clamp is passed bluntly through the rectus muscle.
The open end of the ileal segment is then brought through the hole and everted.
The edge of the stoma is sutured to the abdominal wall with 3-0 interrupted absorbable sutures.
The wound is then irrigated, and a suction drain is placed in the abdomen.
Closure is routine, as described for a laparotomy.
The approach used for the ileal conduit is illustrated in Figure 26-24.
Ileal conduit diagram
Ileal conduit techniques
1. The bowel is mobilized to free a section of ileum.
2 The ileum is transected, and the proximal ileal segment and mesentery are closed.
3 The distal and proximal sections of the ileum are anastomosed.
4 The ureters are implanted into the ileal pouch.
5 A stoma is created in the abdomen.
6 A suction drain is placed in the incision, which is closed in layers.