HES 105 Metabolism MT2 2022
0.0(0)
Card Sorting
1/141
Earn XP
Description and Tags
Last updated 4:07 PM on 10/28/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
142 Terms
1
New cards
power-capacity relationship
(Work/time) capacity
The power cap of each metabolic system varies depending on the system, and the demand of the sport
Y axis - W/kg X axis - time
The power cap of each metabolic system varies depending on the system, and the demand of the sport
Y axis - W/kg X axis - time
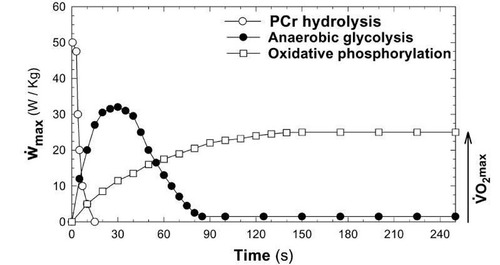
2
New cards
deamination of amino acids
Deamination converts amino acids to a form that can enter energy pathways by removing a nitrogen • Some reduce to pyruvate or other intermediates for the CAC• Happens in the liver - gluconeogenesis
3
New cards
Transamination
Nitrogen passed on from deamination to other compounds
4
New cards
Energy
the capacity to do work
5
New cards
energy transfer
All the chemical processes involved in the production and utilization of ATP
6
New cards
ATP (adenosine triphosphate)
the "common chemical intermediate" for the transfer of energy from food or other high energy compounds to cellular processes including muscle contraction.
• ATP: Adenosine triphosphate
• ATP: Adenosine triphosphate
7
New cards
first law of thermodynamics
Energy can be transferred and transformed, but it cannot be created or destroyed.
Conservation of energy
Conservation of energy
8
New cards
anabolic pathways
Metabolic pathways that consume energy to build complicated molecules from simpler ones.
9
New cards
catabolic pathways
Metabolic pathways that release energy by breaking down complex molecules into simpler compounds.
10
New cards
endergonic reaction
A non-spontaneous chemical reaction in which free energy is absorbed from the surroundings.
11
New cards
exergonic reaction
A spontaneous chemical reaction in which there is a net release of free energy.
12
New cards
second law of thermodynamics
Every energy transfer or transformation increases the entropy of the universe.
Some energy is lost to heat
Some energy is lost to heat
13
New cards
How is ATP stored?
Very small amounts until needed.
Reversible reaction, system in place to re-phosphorylate ATP
Reversible reaction, system in place to re-phosphorylate ATP
14
New cards
Resynthesis of ATP
creatine phosphate, anaerobic glycolysis, oxidative phosphorylation
Using: Glucose/glycogen, Fats, protein
Using: Glucose/glycogen, Fats, protein
15
New cards
Systems approach to energy systems physiology
We can group our energy provision or biochemical pathways into 3 systems:
1. ATP-PCr (Phosphagen)
2. Anaerobic Glycolysis
3. Aerobic System
assists with classifying the energy demands of different activities and types of acute exercise bouts; the demands of different positions in a sport, what energy system is being assessed by an exercise test, and for designing training programs for various reasons.
1. ATP-PCr (Phosphagen)
2. Anaerobic Glycolysis
3. Aerobic System
assists with classifying the energy demands of different activities and types of acute exercise bouts; the demands of different positions in a sport, what energy system is being assessed by an exercise test, and for designing training programs for various reasons.
16
New cards
How much ATP is stored in our muscles at rest?
80-100g
Lasts 1-2s
Lasts 1-2s
17
New cards
ATP-PC system
An energy system that provides energy very rapidly, for approximately 10-15 seconds, via anaerobic metabolism.
Reaction takes place in cytosol (sarcoplasm)
@ high force (0.1-3s
Reaction takes place in cytosol (sarcoplasm)
@ high force (0.1-3s
18
New cards
Steps of the ATP-PCr system
1. ATP stored in the myosin cross-bridges is broken down to release energy for muscle contraction. This leaves the by-products of ATP breakdown: ADP and one single phosphate (Pi)
2. Phosphocreatine (PC) is then broken down by the enzyme creatine kinase into Creatine and Pi
3. The energy released in the breakdown of PC allows ADP and Pi to rejoin forming more ATP. This newly formed ATP can now be broken down to release energy to fuel activity.
2. Phosphocreatine (PC) is then broken down by the enzyme creatine kinase into Creatine and Pi
3. The energy released in the breakdown of PC allows ADP and Pi to rejoin forming more ATP. This newly formed ATP can now be broken down to release energy to fuel activity.
19
New cards
regulators of ATP-PCr system

20
New cards
Controllers of ATP-PCr system

21
New cards
Products of ATP-PCr System
ATP and creatine
22
New cards
ATP-PCr -> myokinase reaction
A reaction in which the enzyme myokinase rapidly replenishes ATP.
ADP + ADP -> ATP + AMP (adenosine monophosphate)
• two molecules of ADP can resynthesize one ATP and can provide a more energy for a few seconds.
• Also important reaction as it recreates the byproducts to start glucose catabolis
ADP + ADP -> ATP + AMP (adenosine monophosphate)
• two molecules of ADP can resynthesize one ATP and can provide a more energy for a few seconds.
• Also important reaction as it recreates the byproducts to start glucose catabolis
23
New cards
adenosine monophosphate (AMP)
a low-energy compound that results from the removal of two phosphate groups from ATP
Can cause nausea
Signal that ATP needs to be synthesized from other sources
Can cause nausea
Signal that ATP needs to be synthesized from other sources
24
New cards
Describe the potential benefits of the ergogenic aid creatine monohydrate
Can increase PCr stores up to 30%
- Greater ability to resynthesize ATP
PCr also shuttles high energy phosphates between mitochondria and cross bridging sites
High levels of PCr are important for all out efforts.
Increasing intramuscular PCr:
• Increase ATP turnover
• Delay depletion
• Decrease dependence on aerobic glycolysis
• Decrease recovery time
- Greater ability to resynthesize ATP
PCr also shuttles high energy phosphates between mitochondria and cross bridging sites
High levels of PCr are important for all out efforts.
Increasing intramuscular PCr:
• Increase ATP turnover
• Delay depletion
• Decrease dependence on aerobic glycolysis
• Decrease recovery time
25
New cards
Creatine loading and maintenance
Protocol: Loading
• Eating large amounts (20 g/day or 0.30 g/kg body mass/day) of Cr over 3 to 5 days for a rapid cellular increase of Cr and smaller increases in PCr.
Protocol: Maintenance
• Maintain loaded levels with continued ingestion of a lower dose (2-5 g/d or 0.03 g/kg/d) for several weeks.
Periodized Plan:
• Load, Maintain, Stop.
• Eating large amounts (20 g/day or 0.30 g/kg body mass/day) of Cr over 3 to 5 days for a rapid cellular increase of Cr and smaller increases in PCr.
Protocol: Maintenance
• Maintain loaded levels with continued ingestion of a lower dose (2-5 g/d or 0.03 g/kg/d) for several weeks.
Periodized Plan:
• Load, Maintain, Stop.
26
New cards
Conditions of creatine supplements
Cr loading may be more effective when taken with carbohydrate (CHO) and less effective if caffeine is ingested simultaneously.
27
New cards
Training power capacity of ATP-PCr system
• Training increases muscles store of high-energy phosphates
• Training can enhance the capacity of the ATP-PCr system
• Not so much on one bout, but after repeated training uses 6-10s repeated maximal efforts
Creatine supplementation is an effective ergogenic aid for training
• Training can enhance the capacity of the ATP-PCr system
• Not so much on one bout, but after repeated training uses 6-10s repeated maximal efforts
Creatine supplementation is an effective ergogenic aid for training
28
New cards
4 supplements that are legal and actually increase performance
1) caffeine
2) creatine
3)
4)
2) creatine
3)
4)
29
New cards
How much are ATP stores reduced after an all out 10s effort?
20%
Phosphocreatine major reduction
Phosphocreatine major reduction
30
New cards
protein metabolism
Deamination -> Transamination
5-10% of total energy for endurance activities could come from amino acids via this process
5-10% of total energy for endurance activities could come from amino acids via this process
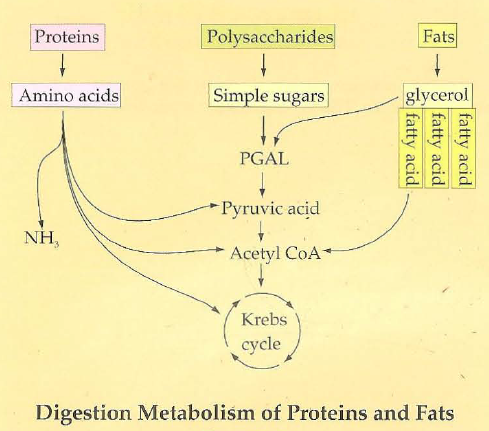
31
New cards
substrates that resynthesize ATP
Creatine kinase
32
New cards
What enzyme catalyzes ATP?
ATP synthase
33
New cards
What enzyme catalyses phosphocreatine and ADP
Creatine Phosphokinase
34
New cards
What substance is essentially more important than ATP for high intensity/short duration exercise?
Creatine
35
New cards
control steps of the glycolytic system
Step 1: Hexokinase (HK):
activating step (uses ATP) to initiate breakdown of glucose.
Step 3: Phosphofructokinase (PFK):
another energy consuming step (uses ATP).
• Major rate limiting enzyme of glycolysis; thus determines the speed of the pathway.
Multivalent, allosteric enzyme which means several things can influence its activity:
• Inhibited by ATP, PCr, citrate, and fl H* (I pH). - slows down process
• Activated by ADP, P,, & AMP. - speeds up process
Phosphorylase:
important enzyme initiating the breakdown of glycogen.
• Activated by Pi, Ca2+ and CAMP (via epinephrine/adrenaline stimulation)
Some Step 10: Pyruvate Kinase
activating step (uses ATP) to initiate breakdown of glucose.
Step 3: Phosphofructokinase (PFK):
another energy consuming step (uses ATP).
• Major rate limiting enzyme of glycolysis; thus determines the speed of the pathway.
Multivalent, allosteric enzyme which means several things can influence its activity:
• Inhibited by ATP, PCr, citrate, and fl H* (I pH). - slows down process
• Activated by ADP, P,, & AMP. - speeds up process
Phosphorylase:
important enzyme initiating the breakdown of glycogen.
• Activated by Pi, Ca2+ and CAMP (via epinephrine/adrenaline stimulation)
Some Step 10: Pyruvate Kinase
36
New cards
Pay off step in glycolysis
Step 7: +2ATP
Step 10: +2ATP
Step 10: +2ATP
37
New cards
The end products of glycolysis are
2 pyruvate, 2 ATP, 2 NADH
• Low-intensity exercise (oxygen present) - pyruvate
• Will enter aerobic metabolic pathways
• Higher-intensity exercise (no oxygen present) -> lactate + H*
Enzyme that catalyzes this reaction is lactate dehydrogenase
• By-product - increased acidity and impairment of contraction - but not bec lactate
• Low-intensity exercise (oxygen present) - pyruvate
• Will enter aerobic metabolic pathways
• Higher-intensity exercise (no oxygen present) -> lactate + H*
Enzyme that catalyzes this reaction is lactate dehydrogenase
• By-product - increased acidity and impairment of contraction - but not bec lactate
38
New cards
Cycling of NADH
Allows glycolysis to proceed at a faster rate
39
New cards
Blood pH after exercise
Blood pH is rarely below 7.0 after exercise but can go as low as 6.74
H+ accumulates in muscle
decrease in ATPase activity so muscle force production and contributes to muscular fatigue.
Remedy by lactate
H+ accumulates in muscle
decrease in ATPase activity so muscle force production and contributes to muscular fatigue.
Remedy by lactate
40
New cards
lactate (La-) in the body
a 3-carbon compound produced from pyruvate during anaerobic metabolism
Influx to tissues
^ oxidation of La- in muscle and other tissues
^ excreted by sweat and urine
Converted to glucose in liver
Influx to tissues
^ oxidation of La- in muscle and other tissues
^ excreted by sweat and urine
Converted to glucose in liver
41
New cards
Lactate is our friend because:
Without lactate we wouldn't be able to exercise at higher intensities for sustained periods
• Pyruvate can not enter the mitochondria without oxygen it picks up a hydrogen ion (via NADH) and forms lactate
• lactate can be shuttled to other parts of the body that do have oxygen
• Hydrogen removed (oxidized) there and it's back to pyruvate
• Lactate can be processed by:
• Other skeletal muscle
• Heart
• Brain
• Liver - cori cycle - gluconeogenesis
• Pyruvate can not enter the mitochondria without oxygen it picks up a hydrogen ion (via NADH) and forms lactate
• lactate can be shuttled to other parts of the body that do have oxygen
• Hydrogen removed (oxidized) there and it's back to pyruvate
• Lactate can be processed by:
• Other skeletal muscle
• Heart
• Brain
• Liver - cori cycle - gluconeogenesis
42
New cards
Recovery from anaerobic glycolysis
Is dependent on managing the pH changes in the muscle and blood caused by the increase H+ production:
43
New cards
lactate dehydrogenase (LDH)
Lactate Dehydrogenase partly determines rate of lactate production
m = muscle form & favors pyruvate to lactate.
h = heart form & favors lactate to pyruvate.
Marathon training increases LDH h and allows athletes to better oxidize lactate for fuel
Sprint training shows increases in LDH m
pyruvate + NADH ↔ lactate + NAD+, present in most tissues, marker of cell damage/death
m = muscle form & favors pyruvate to lactate.
h = heart form & favors lactate to pyruvate.
Marathon training increases LDH h and allows athletes to better oxidize lactate for fuel
Sprint training shows increases in LDH m
pyruvate + NADH ↔ lactate + NAD+, present in most tissues, marker of cell damage/death
44
New cards
Steps of Glycolysis
1. Hexokinase + Glucose(or glycogen) - ATP
2. Glucose-6-phosphate + isomerase
3. Fructose-6-phosphate + Phosphofructokinase - ATP
4. Fructose-1,6-bisphosphate + Aldolase
5. Glyceraldehyde-3-phosphate + Triosephosphate isomerase
6. 1,3-bisphosphoglycerate + Glyceraldehyde
3-phosphate dehydrogenase + NAD+ (to NADH2 to ETChain)
7. 3-phosphoglycerate + Phosphoglycerate kinase + ADP
8. 2-phosphoglycerate + Phosphoglyceromutase
9. Phosphoenolpyruvate + Enolase + H2O
10. Pyruvate + Pyruvate kinase + ATP
2. Glucose-6-phosphate + isomerase
3. Fructose-6-phosphate + Phosphofructokinase - ATP
4. Fructose-1,6-bisphosphate + Aldolase
5. Glyceraldehyde-3-phosphate + Triosephosphate isomerase
6. 1,3-bisphosphoglycerate + Glyceraldehyde
3-phosphate dehydrogenase + NAD+ (to NADH2 to ETChain)
7. 3-phosphoglycerate + Phosphoglycerate kinase + ADP
8. 2-phosphoglycerate + Phosphoglyceromutase
9. Phosphoenolpyruvate + Enolase + H2O
10. Pyruvate + Pyruvate kinase + ATP
45
New cards
power and capacity of the glycolytic system
Moderate to High Power - Moderate to Low Capacity
• Lower peak power output and greater capacity than the ATP-PCr system
• Lower peak power output and greater capacity than the ATP-PCr system
46
New cards
rate of glycolysis
• NAD+/NADH2 Ratio - rate of glycolysis is partly dependent on the availability of NAD* since it is needed for glycolysis to continue to function as a pathway.
• Known as "cycling"
• Substrate Availability - Low glucose/glycogen levels due to fastin disease, improper nutrition, or prior exercise can V the rate of glycolysis/glycogenolysis.
• Known as "cycling"
• Substrate Availability - Low glucose/glycogen levels due to fastin disease, improper nutrition, or prior exercise can V the rate of glycolysis/glycogenolysis.
47
New cards
Glycolysis/lactate system summary
Relies on glucose from blood & glycogen from muscle.
Results in a I in pH of muscle cell (can lead to metabolic acidosis).
Provides moderate to high rate of energy expenditure (power).
Low to Moderate capacity to perform extended high-intensity work.
Fatigue-related by-product (H+) reduces muscle force production.
Results in a I in pH of muscle cell (can lead to metabolic acidosis).
Provides moderate to high rate of energy expenditure (power).
Low to Moderate capacity to perform extended high-intensity work.
Fatigue-related by-product (H+) reduces muscle force production.
48
New cards
Purpose of glycolysis
Main goal is not ATP
Create pyruvate to kick start aerobic metabolism.
Create pyruvate to kick start aerobic metabolism.
49
New cards
Why is glycolysis less productive than the ATP-PCr system
More steps and enzymatic reactions that slow the rate.
50
New cards
Why is glycolysis limited to 90-120s?
Because of the lowering pH as H+ ions are released. Causing metabolic acidosis.
pH will not go below 6.4 ish
pH will not go below 6.4 ish
51
New cards
Who produces more lactate? A sprinter or an endurance athlete? Why?
Sprinter
More fast twitch muscle fibers
More fast twitch muscle fibers
52
New cards
bicarbonate buffer system
CO2 (g)+ H2O(l) ↔ H2CO3 (aq) ↔ H+ (aq)+HCO3- (aq)
-hyperventilation decreases levels of CO2 which causes reaction to shift left consuming H+ and reducing H+ in the blood making pH less acidic
-mechanism that deals w/ acidemia (excess H+ in blood)
most important buffer system that keeps blood pH from changing drastically
-hyperventilation decreases levels of CO2 which causes reaction to shift left consuming H+ and reducing H+ in the blood making pH less acidic
-mechanism that deals w/ acidemia (excess H+ in blood)
most important buffer system that keeps blood pH from changing drastically
53
New cards
sources of H+ in muscle during exercise
Over 90% of the H* generated during anaerobic exercise results from the
high rate of glycolysis.
The remaining 10%:
• ATP. hydrolysis
high rate of glycolysis.
The remaining 10%:
• ATP. hydrolysis
54
New cards
Benefits of bicarbonate buffer system
buffering capacity helps to maintain pH in muscle, extending anaerobic power production or repeated anaerobic power production and reduce power drop off
55
New cards
bicarbonate loading
ingesting large amounts of sodium bicarbonate (1-3 hrs before ) to counteract the effects of lactic acid buildup, thereby reducing fatigue; however, there are potentially dangerous side effects. Don't mess with your blood pH.
56
New cards
Anaerobic training adaptation
1. 1 activity and amount of key glycolytic enzymes [PFK, hexokinase (HK), phosphorylase & LDH (m) form.
• Increases power of system.
2. 1 in skeletal muscle buffering capacity (muscle increases its ability to resist a change in pH).
Increases capacity of system.
3. 11 glycogen stores within muscle.
• Increases capacity of system but also important for power.
• Increases power of system.
2. 1 in skeletal muscle buffering capacity (muscle increases its ability to resist a change in pH).
Increases capacity of system.
3. 11 glycogen stores within muscle.
• Increases capacity of system but also important for power.
57
New cards
The chemical processes of aerobic system
Biochemical pathways at complete the breakdown of glucose/glycogen, fats and some amino acids to produce a large amount of energy to re-phosphorylate ADP to ATP releasing CO, and H,O.
Controller: (glucose, fats)
02+ NADH + ADP + Pi
- >
Products: (amino acids, heat, energy, phosphate)
H2O + CO2 + NAD* + ATP
Controller: (glucose, fats)
02+ NADH + ADP + Pi
- >
Products: (amino acids, heat, energy, phosphate)
H2O + CO2 + NAD* + ATP
58
New cards
Aerobic Glycolysis
• During aerobic exercise up to VO,max intensity, much of the pyruvate produced from glycolysis can enter the mitochondria via a protein channel
• Much of the NADH produced can also be shuttled into the mitochondria:
Via the malate-aspartate (heart) or glycerol phosphate (muscle)
• 2 or 3 ATP are still produced in aerobic glycolysis.
• Pyruvate and NADH are now important products of this system not
• Note that some of the accumulated lactate in muscle can be converted back to pyruvate via LDH(h), reduce NAD* and enter the mitochondria, thus making lactate a fuel for further metabolism and energy
• Much of the NADH produced can also be shuttled into the mitochondria:
Via the malate-aspartate (heart) or glycerol phosphate (muscle)
• 2 or 3 ATP are still produced in aerobic glycolysis.
• Pyruvate and NADH are now important products of this system not
• Note that some of the accumulated lactate in muscle can be converted back to pyruvate via LDH(h), reduce NAD* and enter the mitochondria, thus making lactate a fuel for further metabolism and energy
59
New cards
the chemical processes of the citric acid cycle
• Citric Acid Cycle occurs inside the mitochondria matrix (center).
- Mitochondria is an oval shaped, double membrane organelle within the cell/muscle fiber:
• Once in the mitochondria, pyruvate can be converted to acetyl CoA which enters the Citric Acid Cycle (CAC).
- The Citric Acid Cycle is a circular series of reactions & requires oxaloacetate and acetyl Cod to initiate.
• Main products of the CAC: NADH & FADH2 and 2 ATP resulting from the phosphorylation of ADP from the guanosine triphosphate (GTP) reaction
- Mitochondria is an oval shaped, double membrane organelle within the cell/muscle fiber:
• Once in the mitochondria, pyruvate can be converted to acetyl CoA which enters the Citric Acid Cycle (CAC).
- The Citric Acid Cycle is a circular series of reactions & requires oxaloacetate and acetyl Cod to initiate.
• Main products of the CAC: NADH & FADH2 and 2 ATP resulting from the phosphorylation of ADP from the guanosine triphosphate (GTP) reaction
60
New cards
Preparatory step of CAC
Pyruvate to acetyl CoA
Pyruvate dehydrogenase = CO2 and NADH2 and Acetyl CoA
NOT REVERSIBLE
RATE LIMITING STEP
Pyruvate dehydrogenase = CO2 and NADH2 and Acetyl CoA
NOT REVERSIBLE
RATE LIMITING STEP
61
New cards
pyruvate dehydrogenase
converts pyruvate to acetyl-CoA
Responds to energy needs of the cell by assessing the molecules around it as well as substrate availability.
In general, allosteric activators are usually substrates, and inhibitors are products.
Activated by:
• Pyruvate
• CoA
• NAD
• AMP
• Ca2+
Inhibited by:
• Acetyl CoA
NADH
• ATP
Responds to energy needs of the cell by assessing the molecules around it as well as substrate availability.
In general, allosteric activators are usually substrates, and inhibitors are products.
Activated by:
• Pyruvate
• CoA
• NAD
• AMP
• Ca2+
Inhibited by:
• Acetyl CoA
NADH
• ATP
62
New cards
Step 1 of Citric Acid Cycle
Citrate formation: acetyl-CoA joins with oxaloacetate from a condensation reaction to form citryl-CoA (an intermediate)
Hydrolysis of citryl-CoA -> citrate + CoA-SH
Catalyzed by citrate synthase
Hydrolysis of citryl-CoA -> citrate + CoA-SH
Catalyzed by citrate synthase
63
New cards
Step 2 of Citric Acid Cycle
Citrate is converted to its isomer, isocitrate, by removal of one water molecule and addition of another
64
New cards
Step 3 of Citric Acid Cycle
Alpha-ketoglutarate and CO2 formation: Isocitrate -oxidized by isocitrate dehydrogenase-> oxalosuccinate -decarboxylated-> alpha-ketoglutarate and CO2
Isocitrate dehydrogenase is the rate-limiting enzyme for the citric acid cycle
First C from acetyl-CoA is lost here; first NADH is produced from acetyl-CoA
Isocitrate dehydrogenase is the rate-limiting enzyme for the citric acid cycle
First C from acetyl-CoA is lost here; first NADH is produced from acetyl-CoA
65
New cards
Step 4 of Citric Acid Cycle
Succinyl-CoA and CO2 formation: Carried out by alpha-ketoglutarate dehydrogenase complex, similar in mechanism, cofactors and coenzymes to PDH complex
Alpha-ketoglutarate and CoA come together to form a molecule of CO2 (second and last C lost from acetyl-CoA)
Another NADH is produced by reducing NAD+
Alpha-ketoglutarate and CoA come together to form a molecule of CO2 (second and last C lost from acetyl-CoA)
Another NADH is produced by reducing NAD+
66
New cards
Step 5 of Citric Acid Cycle
Succinate formation:
Succinyl-CoA -hydrolysis of thioester bond-> succinate and CoA-SH
Coupled to phosphorylation of GDP to GTP (driven by energy released from thioester hydrolysis)
Catalyzed by succinyl-CoA synthetase
After GTP is formed, nucleosidediphosphate kinase catalyzes phosphate transfer from GTP to ADP, producing ATP (Only time ATP is produced directly in citric acid cycle)
Succinyl-CoA -hydrolysis of thioester bond-> succinate and CoA-SH
Coupled to phosphorylation of GDP to GTP (driven by energy released from thioester hydrolysis)
Catalyzed by succinyl-CoA synthetase
After GTP is formed, nucleosidediphosphate kinase catalyzes phosphate transfer from GTP to ADP, producing ATP (Only time ATP is produced directly in citric acid cycle)
67
New cards
Step 6 of Citric Acid Cycle
Fumarate formation: Occurs on outer membrane (instead of in mitochondrial matrix)
Succinate -oxidation-> fumarate
Catalyzed by succinate dehydrogenase (a flavoprotein because it is covalently bonded to FAD)
FAD -reduced-> FADH2 -transfers electrons to ETC-> 1.5ATP
Succinate -oxidation-> fumarate
Catalyzed by succinate dehydrogenase (a flavoprotein because it is covalently bonded to FAD)
FAD -reduced-> FADH2 -transfers electrons to ETC-> 1.5ATP
68
New cards
Step 7 of Citric Acid Cycle
Malate formation: Fumarase catalyzes hydrolysis of alkene bond in fumarate, producing malate (only L-malate forms)
69
New cards
Step 8 of Citric Acid Cycle
Oxaloacetate formed again: malate -malate dehydrogenase-> oxaloacetate (oxidation)
3rd NAD+ reduced to NADH
3rd NAD+ reduced to NADH
70
New cards
Which of the following statements is false about allosteric regulation?
a) In general, activators are substrates and inhibitors are products
b) Calcium is an activator in many reactions
c) NAD is an activator in many reactions
d) NADH is an activator in many reactions
a) In general, activators are substrates and inhibitors are products
b) Calcium is an activator in many reactions
c) NAD is an activator in many reactions
d) NADH is an activator in many reactions
D
With lots of NADH typically lots of energy already
With lots of NADH typically lots of energy already
71
New cards
products of citric acid cycle
Per glucose = 6 NADH, 2 FADH2, 2 ATP, 4 CO2
72
New cards
Subdivisions of Aerobic system
Aerobic Glycolysis
Citric Acid Cycle (Kreb/TCA Cycle)
Electron Transport Chain
Beta Oxidation
Citric Acid Cycle (Kreb/TCA Cycle)
Electron Transport Chain
Beta Oxidation
73
New cards
What is the overarching goal of pyruvate?
To enter the mitochondria and initiate the CAC cycle
74
New cards
Which is the best description of oxidative phosphorylation?
The resynthesis of ATP via NADH produced from carbs, fat, or protein oxidation
75
New cards
Summary of aerobic glycolysis
• Pyruvate is end product
• Makes its way into mitochondria via a protein channel
• Once there it starts the citric acid cycle
• Associated H+ get shuttled away to ETC
• Makes its way into mitochondria via a protein channel
• Once there it starts the citric acid cycle
• Associated H+ get shuttled away to ETC
76
New cards
isocitrate dehydrogenase
Responds to energy needs of the cell by assessing the molecules around it as we
substrate availability
Activated by:
• Acetyl CoA
• ADP
•Ca+
Inhibited by:
•ATP
•NADH
substrate availability
Activated by:
• Acetyl CoA
• ADP
•Ca+
Inhibited by:
•ATP
•NADH
77
New cards
Secondary rate limiting enzymes of the CAC
Citrate synthase
Alpha-ketoglutarate dehydrogenase
Alpha-ketoglutarate dehydrogenase
78
New cards
CAC is also where other substrates enter the aerobic system:
• Fats (must go through Beta Oxidation first)
• Amino Acids
• Can also activate CAC
• Amino Acids
• Can also activate CAC
79
New cards
How much ATP from the citric acid cycle?
Per pyruvate: 1ATP + 4NADH + 1FADH2
1 NADH= 3ATP (ETC)
1FADH2 = 2ATP (ETC)
+ 8ATP from aerobic glycolysis
1 NADH= 3ATP (ETC)
1FADH2 = 2ATP (ETC)
+ 8ATP from aerobic glycolysis
80
New cards
Summary of CAC
• Takes place in the mitochondrial matrix
• Citrate synthase turns Acetyl CoA & oxaloacetate into citrate, and then rebuilds
oxaloacetate
• Produces CO2, 1 ATP, 3 NADH, 1 FADH,
NADH and FADH, enter electron transport chain to produce lots of ATP
• Citrate synthase turns Acetyl CoA & oxaloacetate into citrate, and then rebuilds
oxaloacetate
• Produces CO2, 1 ATP, 3 NADH, 1 FADH,
NADH and FADH, enter electron transport chain to produce lots of ATP
81
New cards
Which enzyme is the major rate limiting step in the citric acid cycle:
Isocitrate dehydrogenase
82
New cards
What is the purpose of the citric acid cycle?
generate NADH
Regulated by availability of substrates
Regulated by availability of substrates
83
New cards
The energy system with a moderate to low capacity compared to the two other systems in terms of total ATP production:
Anaerobic glycolysis/glycogenolysis.
84
New cards
Electron Transport Chain (ETC)
A sequence of electron carrier molecules (membrane proteins) that shuttle electrons during the redox reactions that release energy used to make ATP.
• Associated with the inner membrane of the mitochondria.
• Uses NADH* & FADH2 produced in Citric Acid Cycle and Glycolysis:
• The H's (protons) and associated electrons (e-) are stripped off of
NADH/FADH2 & this process spontaneously drives the phosphorylation o
ADP + P. to ATP via the enzyme ATP synthase.
• O2 is utilized at this point and H,O is produced as a by-product.
• Note: FADH, enters ETC at a later stage providing a lower ATP yield compare to
NADH2.
• Associated with the inner membrane of the mitochondria.
• Uses NADH* & FADH2 produced in Citric Acid Cycle and Glycolysis:
• The H's (protons) and associated electrons (e-) are stripped off of
NADH/FADH2 & this process spontaneously drives the phosphorylation o
ADP + P. to ATP via the enzyme ATP synthase.
• O2 is utilized at this point and H,O is produced as a by-product.
• Note: FADH, enters ETC at a later stage providing a lower ATP yield compare to
NADH2.
85
New cards
Oxidative Phosphorylation Regulation
• Based on energy needs of cells (ADP vs ATP stores)
• No allosteric regulation
• Substrate availability
• Cytochrome C oxidase thought to be rate limiting enzyme
• No allosteric regulation
• Substrate availability
• Cytochrome C oxidase thought to be rate limiting enzyme
86
New cards
ATP synthase
Large protein that uses energy from H+ ions to bind ADP and a phosphate group together to produce ATP
87
New cards
Pyruvate
reversible via pyruvate carboxylase
Pyruvate is essential to the speed of the citric acid.
- low pyruvate conc. = glucose/glycogen low (not good)
• Oxaloacetate 'sacrifices' itself to catalyzed back into pyruvate
• Pyruvate goes back to the liver to undergo gluconeogenesis via the Cori cycle
• This replenishes our glucose stores at the expense of slowing down the citric acid cycle due to lowering concentrations of both pyruvate (and in turn acetyl-coA) and oxaloacetate
Pyruvate is essential to the speed of the citric acid.
- low pyruvate conc. = glucose/glycogen low (not good)
• Oxaloacetate 'sacrifices' itself to catalyzed back into pyruvate
• Pyruvate goes back to the liver to undergo gluconeogenesis via the Cori cycle
• This replenishes our glucose stores at the expense of slowing down the citric acid cycle due to lowering concentrations of both pyruvate (and in turn acetyl-coA) and oxaloacetate
88
New cards
key substrates, products and enzymes that regulate beta oxidation
One 16c fatty acid
yield: 7 NADH, 7 FADH, and 8 Acetyl CoA after complete oxidation
Total ATP: 6
Total NADH: 31
Total FADH: 30
----------------
TOTAL ATP (ATP+NADH+FADH) = 129
3 fatty acids in triglyceride therefore 387 ATP
yield: 7 NADH, 7 FADH, and 8 Acetyl CoA after complete oxidation
Total ATP: 6
Total NADH: 31
Total FADH: 30
----------------
TOTAL ATP (ATP+NADH+FADH) = 129
3 fatty acids in triglyceride therefore 387 ATP
89
New cards
Why not rely on fat metabolism?
Fats burn in a Carbohydrate flame
Must have adequate carbs in order to use fat as a fuel source
Pyruvate is necessary for the production of oxaloacetate which is the prep step of the CAC.
• Thus, we must "burn" some CHO (produce some pyruvate) to metabolize fat (& amino acids) as a fuel source.
• If we didn't, fat could not effectively enter the CAC cycle
• Run out of glucose/glycogen > rely more on fat > slow down
Must have adequate carbs in order to use fat as a fuel source
Pyruvate is necessary for the production of oxaloacetate which is the prep step of the CAC.
• Thus, we must "burn" some CHO (produce some pyruvate) to metabolize fat (& amino acids) as a fuel source.
• If we didn't, fat could not effectively enter the CAC cycle
• Run out of glucose/glycogen > rely more on fat > slow down
90
New cards
training adaptations to the aerobic system
1. Substrate level improvements:
2. Oxidative capacity improvements
3. Glycogen Sparing
2. Oxidative capacity improvements
3. Glycogen Sparing
91
New cards
Substrate level training adaptations to the aerobic system
^ glycogen and fat stores in muscle.
^ myoglobin, thus ÝO2 stores in muscle.
^ capillarization (increasing substrate supply and removal of metabolic wastes)
^ myoglobin, thus ÝO2 stores in muscle.
^ capillarization (increasing substrate supply and removal of metabolic wastes)
92
New cards
Oxidative capacity training adaptations to the aerobic system
^ size & number of mitochondria ^ LDH "h" activity.
^ enzyme activities in key pathways and rate limiting enzymes, as well as those responsible for transport across membranes (Fatty acid binding protein).
^ enzyme activities in key pathways and rate limiting enzymes, as well as those responsible for transport across membranes (Fatty acid binding protein).
93
New cards
glycogen sparing training adaptations to the aerobic system
^ availability & rate of oxidation of fats "spares" glycogen from metabolism.
^ use of fatty acids at rest and during submaximal exercise = more glycogen later on
^ use of fatty acids at rest and during submaximal exercise = more glycogen later on
94
New cards
How does availability of substrates limit energy system choice
ATP-PCr (phosphagen system)
• ADP, PCr
Anaerobic Glycolysis
• ADP, Glucose, NAD+•
Aerobic System
• ADP, NAD+, Pyruvate, FAD• NADH + H+, O2
NOTE: High levels of the opposite substrate (i.e., ATP vs. ADP; NADH vs. NAD+) inhibits the activation of energy production
• ADP, PCr
Anaerobic Glycolysis
• ADP, Glucose, NAD+•
Aerobic System
• ADP, NAD+, Pyruvate, FAD• NADH + H+, O2
NOTE: High levels of the opposite substrate (i.e., ATP vs. ADP; NADH vs. NAD+) inhibits the activation of energy production
95
New cards
What Determines/Limits Energy System Choice
O2+ NADH + H++ ADP + Pi àCO2+ H2O + NAD++ ATP
1. Intensity
2. Availability of Substrates
3. Enzyme activity
1. Intensity
2. Availability of Substrates
3. Enzyme activity
96
New cards
How does Intensity limit energy system choice
Rate of ATP turnover
Fast production of ADP will determine Phosphagen vs. Anaerobic Glycolysis vs. Aerobic
Fast production of ADP will determine Phosphagen vs. Anaerobic Glycolysis vs. Aerobic
97
New cards
How does enzyme activity limit energy system choice
ATP-PCr
• Creatine Kinase
Anaerobic Glycolysis
• Hexokinase
• Phosphofructokinase (PFK)
• Lactate dehydrogenase
Aerobic Glycolysis
• Pyruvate dehydrogenase
Aerobic System
• Citrate Synthase
• Isocitrate dehydrogenase
• Cytochrome C oxidase
• Creatine Kinase
Anaerobic Glycolysis
• Hexokinase
• Phosphofructokinase (PFK)
• Lactate dehydrogenase
Aerobic Glycolysis
• Pyruvate dehydrogenase
Aerobic System
• Citrate Synthase
• Isocitrate dehydrogenase
• Cytochrome C oxidase
98
New cards
The general sequence of pathways for glucose metabolism during aerobic endurance exercise is:
a) Glycolysis, beta (B) oxidation, citric acid cycle, electron transport chain
b) Electron transport chain, Beta (R) oxidation, citric acid cycle •
c) Glycolysis, Citric acid cycle, electron transport chain
d) Glycolysis, glycogenolysis, citric acid cycle, electron transport chain
a) Glycolysis, beta (B) oxidation, citric acid cycle, electron transport chain
b) Electron transport chain, Beta (R) oxidation, citric acid cycle •
c) Glycolysis, Citric acid cycle, electron transport chain
d) Glycolysis, glycogenolysis, citric acid cycle, electron transport chain
c)
99
New cards
beta oxidation of fatty acids
Process of taking fatty acyl CoA and forming acetyl CoA
In mitochondrion matrix
In mitochondrion matrix
100
New cards
Gluconeogenesis
The formation of glucose from non carbohydrate sources, such as amino acids.