Module 11: Drug Interactions
5.0(1)
5.0(1)
Card Sorting
1/26
Earn XP
Description and Tags
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
1
New cards
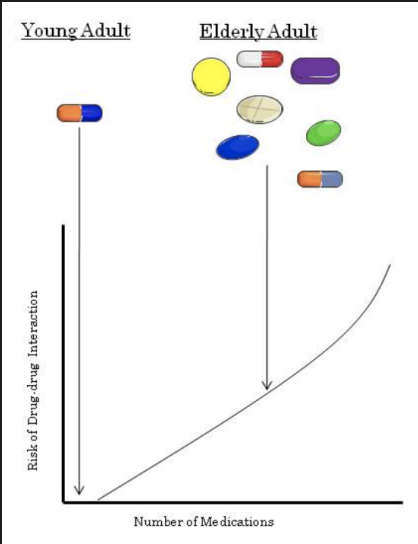
Drug Interactions
* Drugs can interact with each other or components in our diet to produce complicated responses.
* Drug-drug interactions may occur whenever a patient takes two or more drugs.
* Drug-food interactions may occur even if a patient is only taking one drug.
* The risk of drug interactions increases (almost linearly) with the number of medications a patient takes.
* Drug interactions are especially important in the elderly. Why? The average 65 year old takes 7 medications, significantly more than the average 20 year old.
* The most common type of drug-drug interaction are those affecting pharmacokinetics (ADME).
* Drug-drug interactions may occur whenever a patient takes two or more drugs.
* Drug-food interactions may occur even if a patient is only taking one drug.
* The risk of drug interactions increases (almost linearly) with the number of medications a patient takes.
* Drug interactions are especially important in the elderly. Why? The average 65 year old takes 7 medications, significantly more than the average 20 year old.
* The most common type of drug-drug interaction are those affecting pharmacokinetics (ADME).
2
New cards
Consequences of Drug Interactions
* When two drug or more drugs interact there are 3 possible outcomes:
1. Increased effects:
* Drug + Drug → Increased Effect of Drug
2. Decreased effects:
* Drug + Drug → Decreased Effect of Drug
3. Generation of a new effect:
* Drug + Drug → New Effect
1. Increased effects:
* Drug + Drug → Increased Effect of Drug
2. Decreased effects:
* Drug + Drug → Decreased Effect of Drug
3. Generation of a new effect:
* Drug + Drug → New Effect
3
New cards
**Increased effects**
* Drug interactions can increase drug effects by either an increase in the therapeutic effect or an increased adverse effect.
**Increased Therapeutic Effects:**
* Ampicillin is an antibiotic that is rapidly inactivated by bacterial enzymes.
* Sulbactam is an inhibitor of the bacterial enzyme that inactivates ampicillin.
* Ampicillin → Rapid Inactivation, poor therapeutic response
* Ampicillin + Sulbactam → Increased therapeutic activity of ampicillin
**Increased Adverse Effects:**
* Warfarin is an anticoagulant used to thin the blood.
* Aspirin is an analgesic that also thins the blood.
* Warfarin → Effective blood thinner
* Warfarin + Aspirin → Bleeding (potentially life threatening side effect)
**Increased Therapeutic Effects:**
* Ampicillin is an antibiotic that is rapidly inactivated by bacterial enzymes.
* Sulbactam is an inhibitor of the bacterial enzyme that inactivates ampicillin.
* Ampicillin → Rapid Inactivation, poor therapeutic response
* Ampicillin + Sulbactam → Increased therapeutic activity of ampicillin
**Increased Adverse Effects:**
* Warfarin is an anticoagulant used to thin the blood.
* Aspirin is an analgesic that also thins the blood.
* Warfarin → Effective blood thinner
* Warfarin + Aspirin → Bleeding (potentially life threatening side effect)
4
New cards
**Decreased effects**
* Drug interactions can reduce drug effects by either reducing therapeutic effects or reduced adverse effects.
**Reduced Therapeutic Effects:**
* Clopidogrel is an anticoagulant. It is a pro-drug that requires metabolic activation by CYP2C19.
* Omeprazole (a drug used to treat stomach ulcers) inhibits CYP2C19.
* When given together, the active metabolite of clopidogrel is not formed, therefore insufficient anticoagulation occurs.
**Reduced Adverse Effects:**
* Morphine is an analgesic used to treat pain.
* Morphine overdose can produce coma, respiratory depression and even death.
* To treat morphine overdose the competitive antagonist naloxone can be administered.
**Reduced Therapeutic Effects:**
* Clopidogrel is an anticoagulant. It is a pro-drug that requires metabolic activation by CYP2C19.
* Omeprazole (a drug used to treat stomach ulcers) inhibits CYP2C19.
* When given together, the active metabolite of clopidogrel is not formed, therefore insufficient anticoagulation occurs.
**Reduced Adverse Effects:**
* Morphine is an analgesic used to treat pain.
* Morphine overdose can produce coma, respiratory depression and even death.
* To treat morphine overdose the competitive antagonist naloxone can be administered.
5
New cards
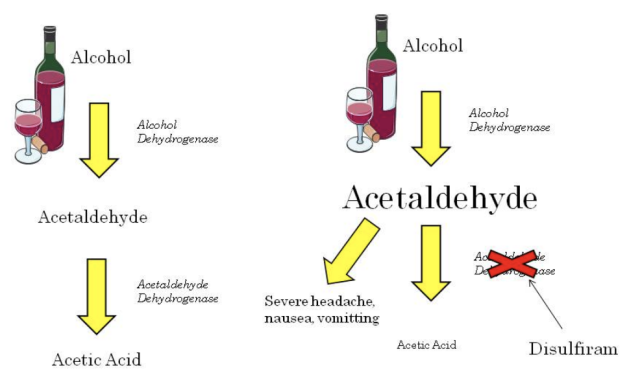
**Generation of a new effect**
* Although drug interactions usually either increase or decrease drug effects, there are a few interactions that produce a unique response.
* Disulfiram is a drug used to help treat chronic alcoholism.
* Alcohol is normally metabolized to acetaldehyde and then further to acetic acid.
* It is the acetaldehyde that makes you feel hungover (headache, nausea, vomiting, visual disturbance etc.).
* Disulfiram inhibits the metabolism of acetaldehyde. Use of this drug causes acetaldehyde levels to increase and patients have very severe hangover like symptoms. This occurs within as little as 10 minutes after alcohol intake.
* Disulfiram is a drug used to help treat chronic alcoholism.
* Alcohol is normally metabolized to acetaldehyde and then further to acetic acid.
* It is the acetaldehyde that makes you feel hungover (headache, nausea, vomiting, visual disturbance etc.).
* Disulfiram inhibits the metabolism of acetaldehyde. Use of this drug causes acetaldehyde levels to increase and patients have very severe hangover like symptoms. This occurs within as little as 10 minutes after alcohol intake.
6
New cards
Types of Drug Interactions
1. Direct Physical Interaction
* Direct chemical or physical interaction of two or more drugs.
2. Pharmacokinetic Interaction
* Interactions affecting absorption, distribution, metabolism or excretion (ADME).
3. Pharmacodynamic Interaction
* Interactions affecting receptor binding.
4. Combined Toxicity
* Two or more drugs exhibit toxicity to the same organ (i.e. hepatotoxicity or QT interval prolongation).
7
New cards
**Direct Physical Interaction**
* The most common direct interaction occurs when two or more IV solutions are mixed together.
* Often mixing IV solutions together can cause a precipitate to form.
* If a precipitate is formed the mixture should be discarded and NOT administered to a patient. “When in doubt, throw it out”.
* Drug solutions should never be mixed together without consulting a compatibility chart.
* The benzodiazepine drug diazepam is particularly problematic and should never be mixed with another drug.
* Although direct drug interactions are more common when mixing medication, they also may occur following administration.
* For example, if a patient is given sodium bicarbonate followed by calcium gluconate, a precipitate may form in the blood.
* Often mixing IV solutions together can cause a precipitate to form.
* If a precipitate is formed the mixture should be discarded and NOT administered to a patient. “When in doubt, throw it out”.
* Drug solutions should never be mixed together without consulting a compatibility chart.
* The benzodiazepine drug diazepam is particularly problematic and should never be mixed with another drug.
* Although direct drug interactions are more common when mixing medication, they also may occur following administration.
* For example, if a patient is given sodium bicarbonate followed by calcium gluconate, a precipitate may form in the blood.
8
New cards
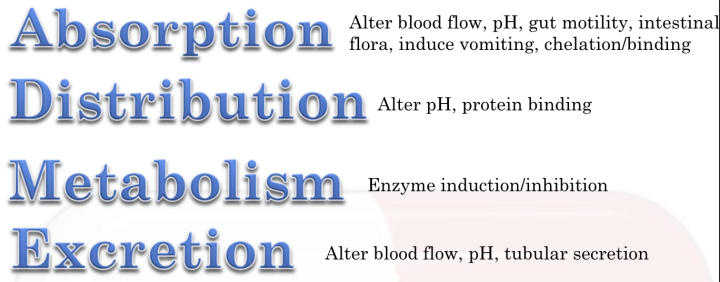
**Pharmacokinetic Interaction**
* Pharmacokinetic drug interactions are by far the most common type of drug interaction experienced in patients.
* In these types of interactions, taking two or more drugs may change the absorption, distribution, metabolism and/or excretion of one or more drugs.
* In these types of interactions, taking two or more drugs may change the absorption, distribution, metabolism and/or excretion of one or more drugs.
9
New cards
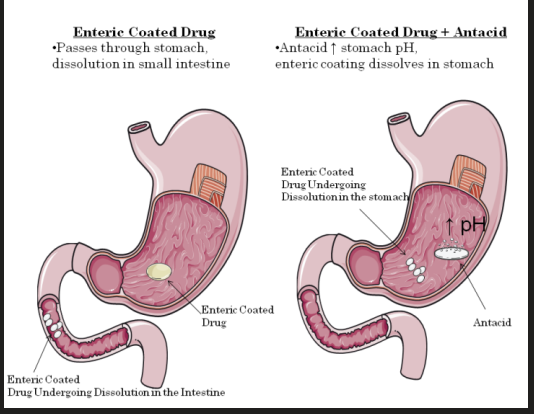
**Absorption - Altered pH**
* We know from modules 1 and 2 that the pH of the environment can have a profound effect on the ionization of many drugs.
* Drugs that effect gastric or intestinal pH can alter drug absorption.
* The most common drug interaction with respect to alteration of pH occurs with antacids.
* Antacids increase gastric pH and therefore increase the absorption of drugs that are weak bases and decrease the absorption of drugs that are weak acids.
* Antacids can also dramatically affect the absorption of enteric coated drugs.
* Enteric coated drugs are designed to pass through the acidic stomach without dissolution. Once they reach the more alkaline intestine dissolution begins.
* When an antacid is taken, it increases the pH of the stomach and therefore promotes premature dissolution of enteric coated drugs.
* Drugs that effect gastric or intestinal pH can alter drug absorption.
* The most common drug interaction with respect to alteration of pH occurs with antacids.
* Antacids increase gastric pH and therefore increase the absorption of drugs that are weak bases and decrease the absorption of drugs that are weak acids.
* Antacids can also dramatically affect the absorption of enteric coated drugs.
* Enteric coated drugs are designed to pass through the acidic stomach without dissolution. Once they reach the more alkaline intestine dissolution begins.
* When an antacid is taken, it increases the pH of the stomach and therefore promotes premature dissolution of enteric coated drugs.
10
New cards
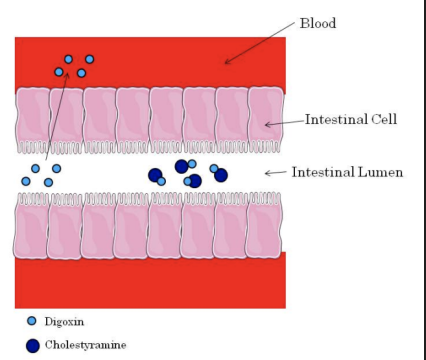
**Absorption - Chelation/Binding**
* Some drugs are known to bind other drugs within the intestine.
* In many cases this binding causes the formation of insoluble complexes that can’t be absorbed.
* The best example of this type of interaction occurs with drugs known as bile acid sequestrants (see Module 12).
* Bile acid sequestrants are designed to bind intestinal bile acids and prevent their absorption from the intestine. The cholestyramine-bile acid complex is excreted in the feces.
* In the figure the bile acid sequestrant cholestyramine binds to digoxin in the intestine and decreases its absorption. Notice how only the free digoxin is absorbed into the blood.
* In many cases this binding causes the formation of insoluble complexes that can’t be absorbed.
* The best example of this type of interaction occurs with drugs known as bile acid sequestrants (see Module 12).
* Bile acid sequestrants are designed to bind intestinal bile acids and prevent their absorption from the intestine. The cholestyramine-bile acid complex is excreted in the feces.
* In the figure the bile acid sequestrant cholestyramine binds to digoxin in the intestine and decreases its absorption. Notice how only the free digoxin is absorbed into the blood.
11
New cards
**Absorption - Altered Blood Flow**
* We know from module 2 that blood flow influences absorption.
* Drugs that decrease blood flow decrease the absorption of drugs.
* One example is the use of epinephrine with a local anesthetic.
* If a local anesthetic is administered alone, it may diffuse into the blood away from the injection site.
* If epinephrine is injected with a local anesthetic, it causes vasoconstriction and decreases the absorption of the local anesthetic. This allows the local anesthetic to stay at the injection site where it is required to prevent pain sensation.
* Drugs that decrease blood flow decrease the absorption of drugs.
* One example is the use of epinephrine with a local anesthetic.
* If a local anesthetic is administered alone, it may diffuse into the blood away from the injection site.
* If epinephrine is injected with a local anesthetic, it causes vasoconstriction and decreases the absorption of the local anesthetic. This allows the local anesthetic to stay at the injection site where it is required to prevent pain sensation.
12
New cards
**Absorption - Gut Motility**
* Some drugs affect intestinal motility.
* Drugs can either cause an increase or a decrease in intestinal motility.
* Laxatives used to treat constipation cause increased gut motility.
* Increased gut motility results in decreased drug absorption.
* Opiate drugs used to treat pain, such as morphine, decrease gut motility and therefore increase drug absorption.
* Drugs can either cause an increase or a decrease in intestinal motility.
* Laxatives used to treat constipation cause increased gut motility.
* Increased gut motility results in decreased drug absorption.
* Opiate drugs used to treat pain, such as morphine, decrease gut motility and therefore increase drug absorption.
13
New cards
**Absorption - Vomiting**
* Drugs that induce vomiting will decrease the absorption of other drugs.
* Patients taking drugs known to have nausea and vomiting as a side effect should be monitored carefully.
* If vomiting occurs within 20 – 30 minutes after taking one or more medications it is likely that absorption is incomplete.
* Health care practitioners must determine whether another dose should be administered.
* If the drug(s) entered the intestine before the patient vomited, giving another dose may produce toxicity.
* Patients taking drugs known to have nausea and vomiting as a side effect should be monitored carefully.
* If vomiting occurs within 20 – 30 minutes after taking one or more medications it is likely that absorption is incomplete.
* Health care practitioners must determine whether another dose should be administered.
* If the drug(s) entered the intestine before the patient vomited, giving another dose may produce toxicity.
14
New cards
**Absorption - Drugs that Kill Intestinal Bacteria**
* Intestinal bacteria play an important role in the metabolism of some drugs.
* Their primary metabolic activity is deconjugating phase II drug metabolites as part of enterohepatic recycling.
* Antibiotic drugs that kill intestinal bacteria can cause decreased deconjugation and therefore decreased absorption during enterohepatic recycling.
* The end result is decreased plasma drug concentration.
* Their primary metabolic activity is deconjugating phase II drug metabolites as part of enterohepatic recycling.
* Antibiotic drugs that kill intestinal bacteria can cause decreased deconjugation and therefore decreased absorption during enterohepatic recycling.
* The end result is decreased plasma drug concentration.
15
New cards
**Distribution - Altering pH**
* A drug that can change extracellular pH can influence the ionization of other drugs.
* Ionization is a major determinant of drug distribution.
* The drug sodium bicarbonate increases the extracellular pH whereas ammonium chloride decreases extracellular pH.
* Due to pH partitioning, changing the extracellular pH can draw a drug from inside the cell to outside by changing its ionization.
* For example, during an overdose of aspirin, increasing the extracellular pH with sodium bicarbonate will draw aspirin (a weak acid) outside the cell and cause it to become ionized. Once “trapped” in the extracellular fluid aspirin may be eliminated in the urine.
* Ionization is a major determinant of drug distribution.
* The drug sodium bicarbonate increases the extracellular pH whereas ammonium chloride decreases extracellular pH.
* Due to pH partitioning, changing the extracellular pH can draw a drug from inside the cell to outside by changing its ionization.
* For example, during an overdose of aspirin, increasing the extracellular pH with sodium bicarbonate will draw aspirin (a weak acid) outside the cell and cause it to become ionized. Once “trapped” in the extracellular fluid aspirin may be eliminated in the urine.
16
New cards
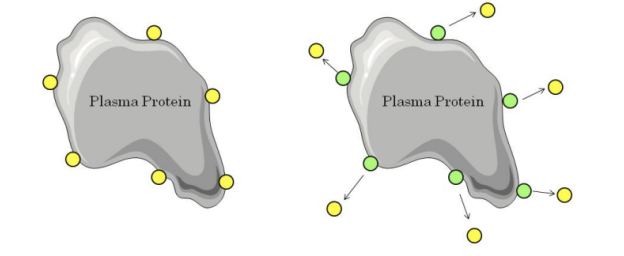
**Distribution - Protein Binding**
* If two drugs are bound to the same site on plasma proteins, co-administration will result in competition for binding.
* The drug with the lower affinity for the protein will become free.
* This may result in increased therapeutic effect, increased toxicity and/or increased excretion.
* In the example below we can see that addition of the green drug causes displacement of the yellow drug from the plasma protein. This causes the free concentration of the yellow drug to increase.
* The drug with the lower affinity for the protein will become free.
* This may result in increased therapeutic effect, increased toxicity and/or increased excretion.
* In the example below we can see that addition of the green drug causes displacement of the yellow drug from the plasma protein. This causes the free concentration of the yellow drug to increase.
17
New cards
**Metabolism - Metabolism**
* Altered drug metabolism is one of the most important and common types of drug interaction.
* Some drugs increase the metabolism of other drugs by inducing drug metabolizing enzymes (CYPs).
* Other drugs inhibit the metabolism of drugs by inhibiting CYPs.
* Most of these types of drug interaction occur in the liver and/or intestine.
* Some drugs increase the metabolism of other drugs by inducing drug metabolizing enzymes (CYPs).
* Other drugs inhibit the metabolism of drugs by inhibiting CYPs.
* Most of these types of drug interaction occur in the liver and/or intestine.
18
New cards
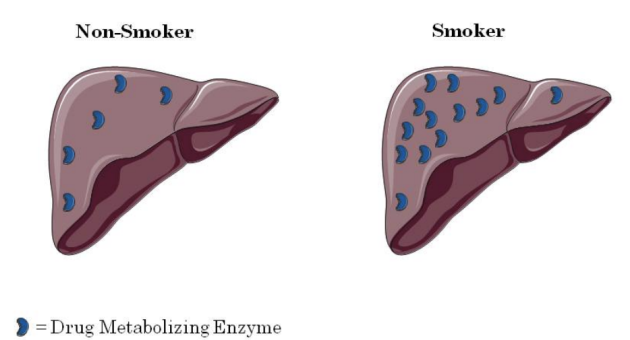
**Metabolism - CYP Induction**
* Some drugs increase the synthesis of CYP enzymes, a process known as induction.
* The result of induction is increased drug metabolism.
* Induction is delayed, therefore it may take 2-10 days following exposure to the enzyme inducer before the induction occurs.
* Once the inducer is stopped it takes 7 – 10 days before the CYP enzyme levels return to normal.
* Examples of CYP inducers include:
* Cigarette/marijuana smoke (one joint has the same effect on induction as 5-10 cigarettes).
* Rifampin – induces CYP3A4
* Phenobarbital – induces many CYPs.
* BBQ’d food – Induces CYP1A2
* Alcohol – Induces CYP2E1 (but severe alcoholism causing cirrhosis decreases CYP activity).
* The result of induction is increased drug metabolism.
* Induction is delayed, therefore it may take 2-10 days following exposure to the enzyme inducer before the induction occurs.
* Once the inducer is stopped it takes 7 – 10 days before the CYP enzyme levels return to normal.
* Examples of CYP inducers include:
* Cigarette/marijuana smoke (one joint has the same effect on induction as 5-10 cigarettes).
* Rifampin – induces CYP3A4
* Phenobarbital – induces many CYPs.
* BBQ’d food – Induces CYP1A2
* Alcohol – Induces CYP2E1 (but severe alcoholism causing cirrhosis decreases CYP activity).
19
New cards
**Metabolism - CYP Inhibition**
* Some drugs can inhibit CYP enzymes in the intestine or liver.
* Enzyme inhibition results in decreased metabolism of other drugs metabolized by the same enzyme.
* Typically enzyme inhibition results in increased plasma concentration of the parent drug.
* If a pro-drug is given when CYP enzymes are inhibited, there will be decreased metabolic activation.
* Examples of CYP inhibitors include:
* Many antibiotics and anti-fungals inhibit CYP3A4.
* HIV protease inhibitors also inhibit CYP3A4.
* Omeprazole inhibits CYP2C19.
* Selective serotonin reuptake inhibitors (SSRIs) – inhibit CYP2D6.
* Fluvoxamine – Inhibits CYP1A2
* Grapefruit juice – Inhibits CYP3A4
* In the figure, drug administered without grapefruit juice is metabolized in the intestine prior to entering the blood. When grapefruit juice is administered it inhibits CYP3A4, therefore higher drug concentrations enter the blood.
* Enzyme inhibition results in decreased metabolism of other drugs metabolized by the same enzyme.
* Typically enzyme inhibition results in increased plasma concentration of the parent drug.
* If a pro-drug is given when CYP enzymes are inhibited, there will be decreased metabolic activation.
* Examples of CYP inhibitors include:
* Many antibiotics and anti-fungals inhibit CYP3A4.
* HIV protease inhibitors also inhibit CYP3A4.
* Omeprazole inhibits CYP2C19.
* Selective serotonin reuptake inhibitors (SSRIs) – inhibit CYP2D6.
* Fluvoxamine – Inhibits CYP1A2
* Grapefruit juice – Inhibits CYP3A4
* In the figure, drug administered without grapefruit juice is metabolized in the intestine prior to entering the blood. When grapefruit juice is administered it inhibits CYP3A4, therefore higher drug concentrations enter the blood.
20
New cards
**Excretion - Altered Blood Flow**
* Drugs that decrease renal blood flow cause a decrease in glomerular filtration.
* Decreased glomerular filtration causes decreased renal excretion which will cause increased plasma drug concentrations.
* Non-steroidal anti-inflammatory drugs (NSAIDS) used as analgesics cause renal vasoconstriction and therefore decrease renal blood flow.
* Beta blockers act on the heart to decrease cardiac output. Decreased cardiac output indirectly decreases renal blood flow.
* Decreased glomerular filtration causes decreased renal excretion which will cause increased plasma drug concentrations.
* Non-steroidal anti-inflammatory drugs (NSAIDS) used as analgesics cause renal vasoconstriction and therefore decrease renal blood flow.
* Beta blockers act on the heart to decrease cardiac output. Decreased cardiac output indirectly decreases renal blood flow.
21
New cards
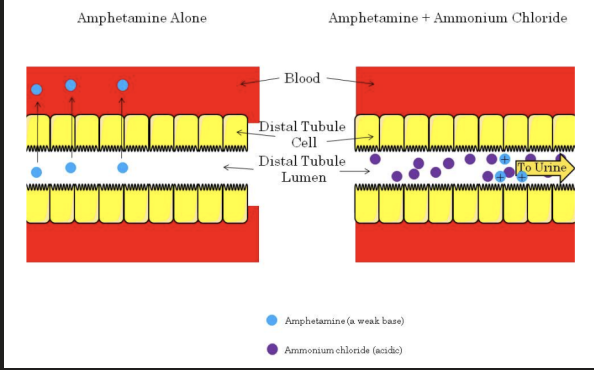
**Excretion - Altered pH**
* Drugs that change the pH of the renal tubular filtrate can alter drug excretion.
* This is due to pH partitioning and ion trapping.
* Altering the renal tubular filtrate can be a useful drug interaction in terms of the treatment of drug overdose.
* If a patient overdoses on amphetamine (a weak base), the filtrate may be acidified with ammonium chloride.
* This causes the amphetamine to be ionized and therefore trapped in the renal tubules and prevents reabsorption into the blood.
* The net result is increased renal excretion of the amphetamine.
* This is due to pH partitioning and ion trapping.
* Altering the renal tubular filtrate can be a useful drug interaction in terms of the treatment of drug overdose.
* If a patient overdoses on amphetamine (a weak base), the filtrate may be acidified with ammonium chloride.
* This causes the amphetamine to be ionized and therefore trapped in the renal tubules and prevents reabsorption into the blood.
* The net result is increased renal excretion of the amphetamine.
22
New cards
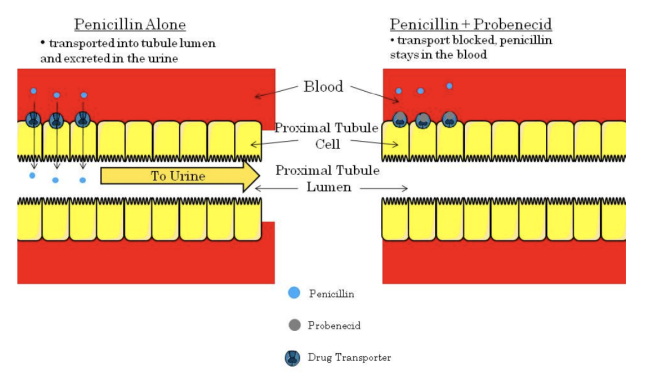
**Excretion - Tubular Secretion**
* Tubular secretion is mediated by transporters at the proximal tubule.
* If one drug blocks the transporter, it may block another drug from being secreted into the tubule lumen.
* This will ultimately result in decreased renal excretion and increased plasma concentration.
* The best example of a drug interaction involving secretion involves the antibiotic penicillin and the drug probenecid (used to treat gout).
* When administered together, probenecid inhibits the transporter responsible for moving penicillin from the blood into the tubule lumen.
* This causes renal penicillin excretion to decrease and blood penicillin concentration to rise.
* This interaction was intentionally used during times of war when penicillin supplies were low.
* If one drug blocks the transporter, it may block another drug from being secreted into the tubule lumen.
* This will ultimately result in decreased renal excretion and increased plasma concentration.
* The best example of a drug interaction involving secretion involves the antibiotic penicillin and the drug probenecid (used to treat gout).
* When administered together, probenecid inhibits the transporter responsible for moving penicillin from the blood into the tubule lumen.
* This causes renal penicillin excretion to decrease and blood penicillin concentration to rise.
* This interaction was intentionally used during times of war when penicillin supplies were low.
23
New cards
**Pharmacodynamic Interaction**
There are two main types of pharmacodynamic drug interactions:
1. Interactions that occur at the same receptor.
2. Interactions that occur at separate sites.
1. Interactions that occur at the same receptor.
2. Interactions that occur at separate sites.
24
New cards
**Interactions that occur at the same receptor**
* Usually drug interactions that occur at the same receptor are the result of an antagonist blocking the action of an agonist (i.e. inhibitory).
* Drug interactions at the same receptor site can cause decreased therapeutic action.
* They can also decrease toxicity in overdose situations. This is a beneficial interaction.
* For example, when a patient overdoses on morphine they have the symptoms of respiratory depression and coma.
* In this situation naloxone, a competitive antagonist can be administered to reverse the symptoms.
* Drug interactions at the same receptor site can cause decreased therapeutic action.
* They can also decrease toxicity in overdose situations. This is a beneficial interaction.
* For example, when a patient overdoses on morphine they have the symptoms of respiratory depression and coma.
* In this situation naloxone, a competitive antagonist can be administered to reverse the symptoms.
25
New cards
**Interactions that occur at separate sites**
* Two drugs that have completely different mechanisms can produce a drug interaction if they produce the same physiological response.
* For example, the analgesic morphine and the anxiolytic drug diazepam are both central nervous system depressants.
* Although they both act in the brain they bind to completely different receptors to produce their effects.
* Morphine acts on brain opioid receptors whereas diazepam acts on the benzodiazepine receptor.
* When these drugs are taken together, the result is enhanced CNS depression.
* For example, the analgesic morphine and the anxiolytic drug diazepam are both central nervous system depressants.
* Although they both act in the brain they bind to completely different receptors to produce their effects.
* Morphine acts on brain opioid receptors whereas diazepam acts on the benzodiazepine receptor.
* When these drugs are taken together, the result is enhanced CNS depression.
26
New cards
**Combined Toxicity**
* This one is a little more obvious than the others!
* If you know one drug may cause hepatotoxicity, would you give a patient another drug that may also cause hepatotoxicity? This would almost certainly produce a drug interaction known as combined toxicity.
* Examples of combined toxicity include the use of acetaminophen and alcohol. Both are hepatotoxic and when used together can increase the amount of liver damage.
* Isoniazid and rifampin are both used to treat tuberculosis and are both hepatotoxic. Unfortunately effective treatment of tuberculosis requires both drugs. In a situation like this liver function tests must be performed to monitor the degree of hepatotoxicity.
* If you know one drug may cause hepatotoxicity, would you give a patient another drug that may also cause hepatotoxicity? This would almost certainly produce a drug interaction known as combined toxicity.
* Examples of combined toxicity include the use of acetaminophen and alcohol. Both are hepatotoxic and when used together can increase the amount of liver damage.
* Isoniazid and rifampin are both used to treat tuberculosis and are both hepatotoxic. Unfortunately effective treatment of tuberculosis requires both drugs. In a situation like this liver function tests must be performed to monitor the degree of hepatotoxicity.
27
New cards
Food - Drug Interactions
* We have already discussed the importance of the interaction between grapefruit juice and CYP3A4.
* Another important food drug interaction exists with monoamine oxidase (MAO) inhibitors, a class of drug used to treat depression.
* Patients taking these drugs must avoid foods containing tyramine including aged cheese, yeast, red wine, sauerkraut, cured meat (i.e. pepperoni) and soy sauce.
* MAO inhibitors inhibit the breakdown of tyramine.
* Tyramine causes increased release of norepinephrine from peripheral nerve terminals resulting in a potentially fatal hypertensive crisis.
* Symptoms of hypertensive crisis include tachycardia (increased heart rate), severe hypertension, headache, nausea and vomiting.
* MAO inhibitors should not be used in patients not capable of adhering to strict dietary restrictions.
* Another important food drug interaction exists with monoamine oxidase (MAO) inhibitors, a class of drug used to treat depression.
* Patients taking these drugs must avoid foods containing tyramine including aged cheese, yeast, red wine, sauerkraut, cured meat (i.e. pepperoni) and soy sauce.
* MAO inhibitors inhibit the breakdown of tyramine.
* Tyramine causes increased release of norepinephrine from peripheral nerve terminals resulting in a potentially fatal hypertensive crisis.
* Symptoms of hypertensive crisis include tachycardia (increased heart rate), severe hypertension, headache, nausea and vomiting.
* MAO inhibitors should not be used in patients not capable of adhering to strict dietary restrictions.