Reagants for Organic Chemistry
1/65
Earn XP
Description and Tags
Mane marvu chhe; quiero morir
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
SN1 Characteristics
Polar protic acid
Weak nucleophile
Forms carbocation —> rearrangement
Forms racemic mixture
Prefers tertiary and secondary substrates.
Bu3SnH, AlBn + heat
Replaces halide (Br, Cl, I) with H.
Benzyolperoxide or AlBN + heat
Polymerizes alkenes via free radical mechanism
SN2 Characteristics
Polar aprotic solvent
Strong nucleophile
No carbocation rearrangement
Stereochemical inversion (backside attack)
Prefers primary and secondary over tertiary substrates
TsCl, NEt3 or Pyridine
Converts alcohols into OTs, a good leaving group. Does not change configuration.
TfCl, NEt3 or Pyridine
Converts alcohols into OTf, a better leaving group than OTs. Does not change configuration.
MsCl, NEt3 or Pyridine
Converts alcohols into OMs, a good leaving group. Does not change configuration.
PBr3
Converts alcohols into bromides. Inverts configuration. (SN2)
SOCl2
Converts alcohols into chlorides. Inverts configuration. (SN2)
Mitsunobu: PPh3, DEAD, THF, and and HX (can be RCOOH, PhOH, RSH, HCN, HN3).
Converts alcohols into X (ex: RCOO, OPh, RS, CN, N3). Inverts configuration.
Williamson Ether Synthesis (NaH, NaNH2, or NaOH + CH3Br)
Deprotonates alcohol to form alkoxide, which then undergoes nucleophilic substitution with an alkyl halide to form an ether.
If intramolecular (OH on one end and leaving group on other end) then forms cyclic ether.
Ether + Strong Acid (HBr, HI)
Strong acid attacks least hindered sp3 carbon adjacent to O and cleaves ether.
Ether + (CH3)3SiI
(CH3)3SiI attacks least hindered sp3 carbon adjacent to O and cleaves ether.
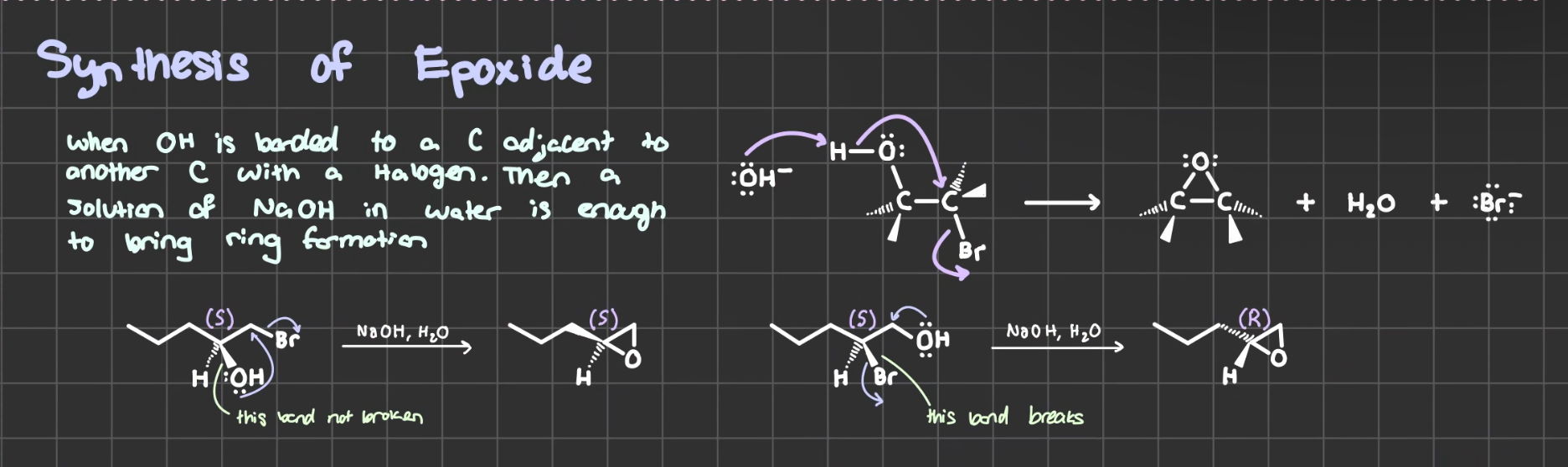
NaOH, H2O (OH bonded to Carbon adjacent to another Carbon with a halogen)
Synthesizes an epoxide. Stereochemistry may invert.
If leaving group is solid line, bond is not broken and stereochemistry does not change.
If leaving group is wedge or dash, bond is broken and stereochemistry inverts.
Epoxide Opening in Acidic Conditions
SN1. Nucleophile attacks more substituted carbon. Acid is usually H2SO4 or HX.
Epoxide Opening in Basic Conditions
SN2. Nucleophile reacts with less substituted carbon. Strong nucleophile (basic).
Thiols (RSH) + NEt3
Forms thioethers (R-S-R). Inverts stereochemistry (SN2)
Thiols (RSH) + OH-
Forms thioethers (R-S-R). Forms carbocation after leaving group leaves. May undergo rearrangement before adding thiol. (SN1)
Thiols + PPh3, DEAD, THF
Thiols can act as nucleophile in Mitsunobu. Inverts stereochemistry. SN2.
H2 + Pd-C/Pd/Pt/Ni
Syn addition
Reduces aldehydes and ketones on ring
Used to add H atoms
CN → CH2NH2
N3 → NH2
Ph-NO2 → Ph-NH2
Ph-COR’ —> Ph-CH2-R’
H2 + Pd-C (Lindlar’s Catalyst)
Converts alkynes into cis alkenes (Partial reduction)
2H2 + Pd-C or other catalyst (Pt, Ni, Pd)
Converts alkynes into alkanes (complete reduction)
Na, NH3 (l), -33 degrees Celsius
Converts alkynes into trans alkenes.
NaCN + DMF
Replaces leaving group (usually Br, I, Cl) with CN and inverts stereochemistry.
NaBH4 + protic solvent (H2O or CH3OH)
Reduces carbonyls (aldehydes and ketones).
Ketones → secondary alcohols
Aldehydes → primary alcohols
O3 + (CH3)2S
Converts alkene into aldehyde and ketone
O3 + H2O2
Converts alkene into carboxylic acid and ketone
OsO4 + t-BuOOH + t-BuOH + OH-
Syn addition
Converts alkene into diol (two OH groups; one at each end of double bond)
If cis alkene → meso
If trans alkene → enantiomers (racemic)
HIO4 or NaIO4, H2O
Vicinal diols (two OH adjacent OH) are cleaved and form aldehydes and/or ketones
RCO3H or MCPBA + CH2Cl2
Converts alkene into epoxide
Syn addition
Cis alkene → meso
Trans alkene → enantiomers (racemic)
H2CrO4, H2O, acetone
H2SO4, acetone (Jones reagant)
Na2Cr2O7, H2SO4, H2O
CrO3, H2SO4
These all oxidize primary and secondary alcohols
In primary alcohols, forms aldehyde, then can oxidize again into carboxylic acid
In secondary alcohols, forms ketones
CrO3, Pyr (Collen’s Reagant)
Stops reaction of oxidation of primary alcohols at aldehyde
PCC + CH2Cl2 (Corey’s Reagant)
Stops reaction of oxidation of primary alcohols at aldehyde
DMSO, COCl2, Et3N
Stops reaction of oxidation of primary alcohols at aldehyde. Less toxic.
IBX in DMSO
Oxidizes alcohol group without affecting other functional groups.
DMP in CH2Cl2
Oxidizes alcohol group without affecting other functional groups.
MnO2
Oxidizes benzylic and allylic alcohols to aldehydes and ketones
RCO3H + H2O2
Oxidizes tertiary amine (it just adds O)
H-X (HBr, HI, HCl) added to alkene
Forms carbocation → can rearrange.
Markovnikov addition
Rate = k[alkene][HX]
Regioselective (tertiary > secondary > primary)
Adds X (Br, I, Cl) to more substituted carbon in alkene
SN1 → forms racemic mixture
H-X (HBr, HI, HCl) added to alkene in presence of ROOR
DOES NOT FORM carbocation
Anti-Markovnikov addition
Adds X (Br, I, Cl) to less substituted carbon in alkene
SN1 → forms racemic mixture
H2O + H2SO4
Forms carbocation → can rearrange
Markovnikov addition
Regioselective (tertiary > secondary > primary)
Adds OH to more substituted carbon in alkene.
SN1 → forms racemic mixture
ROH + H2SO4
Forms carbocation → can rearrange
Markovnikov addition
Regioselective (tertiary > secondary > primary)
Adds ROH to more substituted carbon in alkene.
SN1 → forms racemic mixture
Hg(OAc)2 + H2O + THF (Oxymercuration)
Does not form carbocation
Markovnikov addition
Anti addition (HgOAc and OH on different sides)
Forms triangle thing. H2O attacks more substituted carbon and then gets deprotonated
HgOAc always on less substituted end and OH always on more substituted end.
NaBH4 (Demercuration)
Replaces HgOAc with H after oxymercuration
Hg(OAc)2 + ROH + THF (Oxymercuration)
Does not form carbocation
Anti addition (HgOAc and ROH on different sides)
Forms ether due to ROH
Markovnikov addition
Forms triangle thing. ROH attacks more substituted carbon and then gets deprotonated
HgOAc always on less substituted end and ROH always on more substituted end.
BH3, THF
H2O2, OH-
Does not form carbocation
Syn addition
Anti-markovnikov addition
H on more substituted C and OH on less substituted C
If trans, forms enantiomers
If cis, forms meso compound.
X2, CH2Cl2 (X2 = Br2, Cl2) in alkenes
No carbocation
Anti addition
Both X are added and have different configurations
Markovnikov addition
Forms triangle thing
X attacks more substituted carbon
If cis, forms enantiomers
If trans, forms meso compound
If bulky substituent, only one product
X2, H2O (X2 = Br2, Cl2) in alkenes
No carbocation
Anti addition
Markovnikov addition.
Forms triangle thing
OH attacks more substituted carbon and gets deprotonated
Forms enantiomers
H-X + alkyne (X = Br, Cl)
Converts alkynes into alkenes,
IF EXCESS: converts alkene into alkane
X2 (Br2, Cl2) in alkynes
No carbocation
Anti addition.
Forms trans alkene
Markovnikov addition
Forms triangle thing
X attacks more substituted carbon
IF EXCESS: trans alkene forms alkane
HgSO4 + H2SO4 + H2O
OR
PtCl2 + H2O
Converts internal alkynes into ketones and terminal alkynes into methyl ketones
OH attached on more substituted C (enol)
Both: Alkyne → enol → ketones (resonance)
(Sia)2BH, THF
H2O2, OH-
Does not form carbocation
Syn addition
Anti-markovnikov addition
H on more substituted C and OH on less substituted C
If trans, forms enantiomers
If cis, forms meso compound.
Stops at alkene (enol), but can become ketone through keto-enol tautomerism
H2SO4 or HCOOH + alkene
Forms C-C bonds (Polymerization)
H2SO4 or HCOOH + alkene with OH
Forms C-C bonds. Forms ring if possible
Anything with Zn, CH2I2 + Alkene
Forms triangle thing
If cis, stereochemistry is same
If trans, stereochemistry is different
CHCl3 + Alkene
Forms triangle thing with two Cl coming out of the point (solid line).
If cis, stereochemistry of other ends are same
If trans, stereochemistry if other ends are different
E1 for Alcohol
H2SO4 or H2PO4 + Heat
Acid protonates alcohol, OH2 leaves, forms carbocation, rearrangement, Beta hydrogen forms alkene.
Favors tertiary and secondary carbocations. NEVER primary
Forms carbocation
More substituted alkene is more stable
E1 for Alkyl Halide
CH3OH, heat
Leaving group leaves, carbocation forms, rearrangement, beta hydrogen forms alkene
Favors tertiary and secondary carbocations
Forms carbocation
More substituted alkene is more stable
Weak base
Polar protic solvent + heat
Competing with SN1
E2 for Alcohol
H2SO4 or H2PO4 + Heat
MUST BE anti-periplanar
ONLY Primary alcohol
HIGH HEAT REQUIRED
E2 for Alkyl Halide
One step. Base attacks beta hydrogen and forms alkene and leaving group leaves all at once.
Antiperiplanar
Strong base (OH, OR, NH3, LDA)
Polar protic solvent + heat
Can work with primary, secondary, and tertiary alkyl halides
SnCl2, HCl
Ph-NO2 —> Ph-NH2
Zn, HOAc
Ph-NO2 —> Ph-NH2
Zn(Hg), HCl
Ph-NO2 —> Ph-NH2
Ketone (RCOR’) —> methylene (RCH2-R’)
H2SO4 + H2O + heat
Ph-SO3H —> Benzene
NH2NH2 + KOH + heat
Reduces ketones into methylene
Ketone (RCOR’) —> methylene (RCH2-R’)
(CH3CO)2O
Converts aniline into acetanilide. Used to get a monobromination product.