Benzene
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
Kekule’s proposed structure
cyclohexatriene
High ratio of C:H suggests highly unsaturated molecule, but benzene is not reactive
3 pieces of evidence to discount Kekules structure
X-ray Crystallography - Shows benzene has 6 equal length C-C bonds
Mechanism - With 3 double bonds it should react by electrophilic addition but it reacts via subsitution
Thermochemical evidence from ΔΗhydrogenation - When hydrogenating cyclohexene into cyclohexane, ΔΗ is 120kJ/mol, so hydrogenation of benzene should be 3x higher, but it is a lot lower showing that it is more stable than expected
Nitration of benzene
Generation of electrophile
Electrophilic substitution
Regeneration of the catalyst
C6H6 + HNO3 + H2SO4 → C6H5NO2
Concentrated HNO3 and concentrated H2SO4 (catalyst)
Generation of electrophile in nitration
HNO3 + 2H2SO4 → +NO2 + H3O+ + 2HSO4-
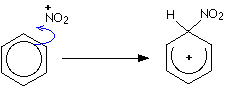
Electrophilic substitution in nitration
Makes nitrobenzene which can be reduced into phenylamine
Regeneration of catalyst in nitration
H3O+ + 2HSO4- + H+ → 2H2SO4 + H2O
Friedel-Craft Acylation
Acyl group is substituted for a ‘H’ on benzene ring
Generation of electrophile
Electrophilic substituion
Regeneration of catalyst
Generation of electrophile in Friedel-Craft
Acid chloride + AlCl3 → RCO+ + AlCl4-
AlCl3 = catalyst, halogen carner
Electrophilic substitution
Makes ketone structure

Regeneration of catalyst in Friedel-Craft
AlCl4- + H+ → AlCl3 + HCl