Pathophysiology II - Exam 4 - Pain 😖
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
T/F: pain is objective
FALSE
- pain is subjective
- common reason for medical attention, but is often undertreated
- cause can be obvious OR unknown
what is the pain threshold?
the lowest point at which a stimulus is perceived as pain
what is pain tolerance?
maximum intensity or duration a person can endure WITHOUT intervention
- dependent on psychological, familial, cultural, environmental, and physiological factors
define the following terms related to alterations in pain sensitivity:
- hyperalgesia
- allodynia
- paresthesia
- analgesia
- hyperalgesia: increased sensitivity to pain
- allodynia: pain resulting from stimuli which is NOT normally painful
- paresthesia: "pins and needles"
- analgesia: pain relief
what is nociceptive pain? what are the 3 primary causes?
pain from noxious stimuli
- temperature extremes
- mechanical trauma
- chemical irritation
often associated with healing!
what are the 2 classes of nociceptive pain?
somatic
- skin, bone, joint, muscle, or CT involvement
- presents as sharp, throbbing, well-localized pain (patient can often point to the exact location of pain)
visceral
- internal organ involvement
- may manifest as poorly-localized, referred pain
- deep, aching, colicky
what is adaptive pain?
pain that occurs from tissue damage, which engages the immune system and causes inflammation
- increased pain sensitivity in the inflamed area
- result is decreased touch and movement (promotes healing)
with adaptive pain, changes in NTs and nerves may alter...?
transmission and transduction
- results in a hypersensitivity state
what is pain transduction?
the stimulation of pain nerve fibers (nociceptors)
- found in both somatic and visceral locations
- distinguishes between noxious and innocuous stimuli
- noxious stimuli activate cytokines and chemokines that sensitize nociceptors
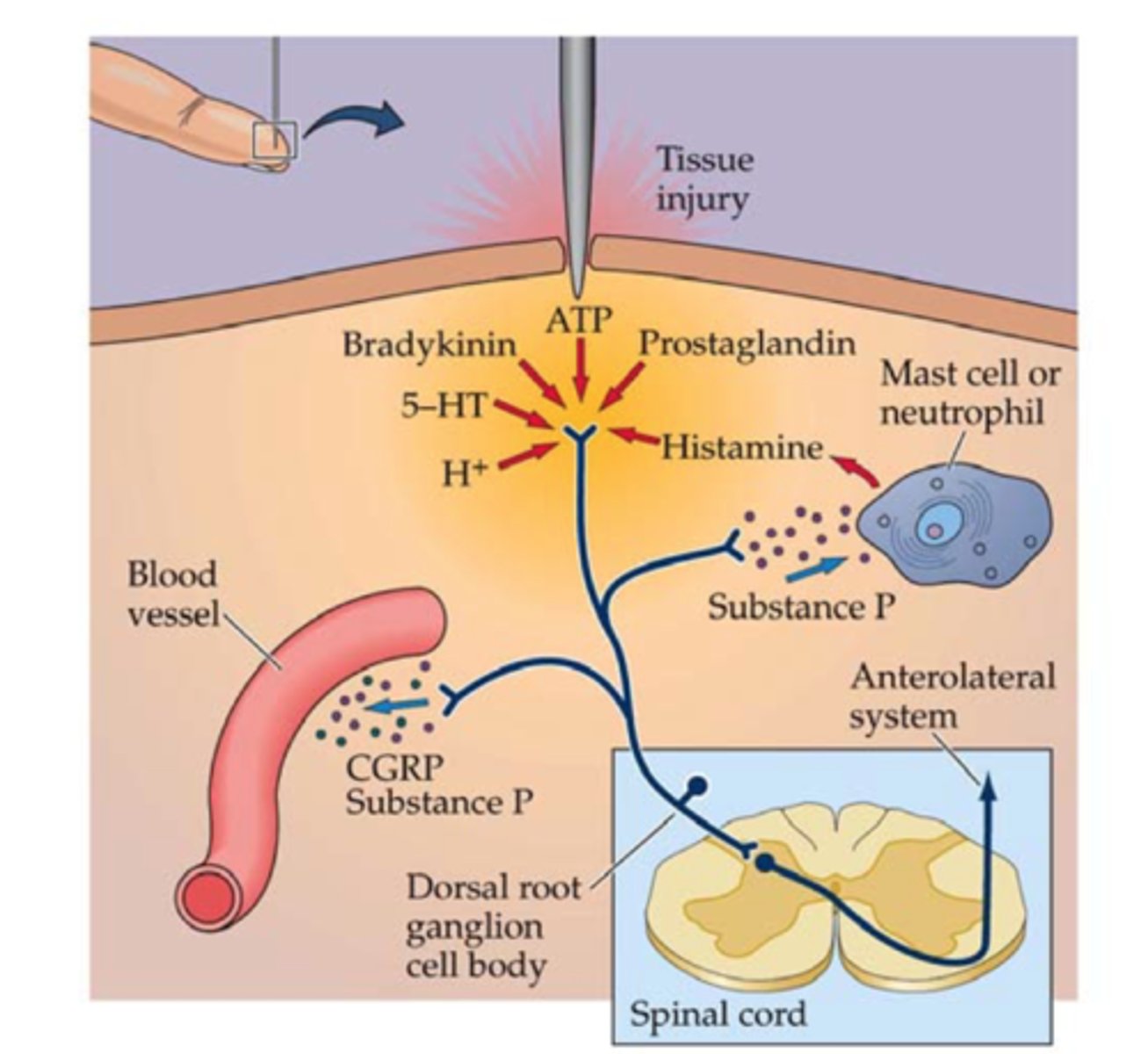
what is pain conduction?
nociceptor activation
- conversion of a chemical signal into an electrical signal
- requires voltage-gated Na+ channels
- action potentials are conducted on primary afferent nerves to the dorsal horn of the spinal cord
what are the 2 types of afferent nerves involved in pain conduction? what type of pain is modulated by each?
- Aδ: produces sharp, localized pain
- C-polymodal: produces aching, poorly-localized pain
what is pain transmission?
afferent nerve fibers synapse in the laminae of the spinal cord dorsal horn
- convert electrical signals back into chemical signals
- the transport of pain signals to the brain
how do afferent nerve fibers convert electrical signals back into chemical signals during pain transmission? what regulates this process?
release excitatory NTs, including glutamate and substance P
- regulated by N-type voltage-gated Ca2+ channels
pain transmission - the ________________ acts a relay center within the brain
thalamus
- passes pain impulses to higher cortical structures
what is pain modulation? what substances increase or decrease pain?
mechanisms that affect the transmission or perception of pain
- increase pain: NTs like glutamate and substance P
- decrease pain: descending pathways
■ endogenous opiate system (endorphins, enkephalins, dynorphins)
■ GABA
■ NE
■ 5-HT
■ blockage of NMDA Rs
what is pain perception? what modifications can alter perception?
when pain becomes a conscious experience
- higher cortical structures are involved (thalamus)
- cognitive and behavioral modifications can alter perception and limit pain signals
what is maladaptive (pathologic) pain?
pain with NO noxious stimuli
- not necessarily associated with healing
- often chronic
- results from abnormal functioning of the PNS or CNS
describe the 2 classes of maladaptive pain
neuropathic
- ongoing peripheral OR central NS injury
- ex: postherpetic neuralgia, chemo-induced neuropathy (peripheral) / stroke, MI (central)
centralized
- NO nerve injury, but is instead caused by a disturbance in pain processing
- ex: fibromyalgia, IBS, myofascial pain syndrome
the mechanism of neuropathic and functional pain is due to what types of changes in the NS?
due to dynamic changes in the NS
- inflammatory changes
- ectopic excitability
- enhanced transmission
- nerve structure reorganization
- loss of inhibitory pain modulators
- rewiring of pain circuits (mismatch between inhibition and stimulation causes progressive discharge from neurons)
describe the clinical presentation of neuropathic and functional pain
episodic or continuous burning, tingling, or shooting pain
- allodynia
- often persists AFTER healing has occurred
- can either affect a large area (like DM) or local area (tumor, neuralgia)
what is neuralgia?
throbbing or "lightning-like" pain along the distribution of a nerve
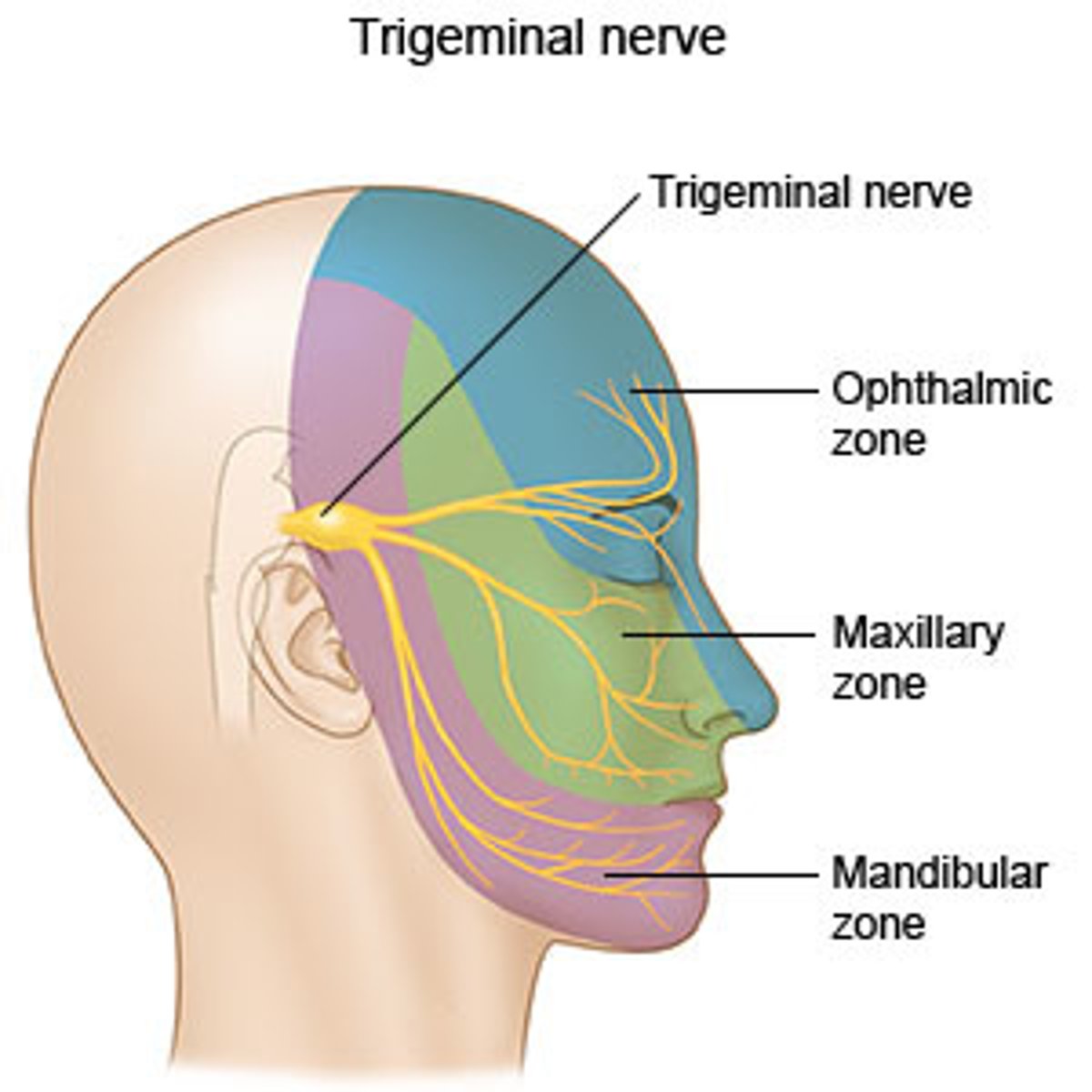
what is trigeminal neuralgia?
neuralgia of the fascial nerve (trigeminal)
- causes facial tics or spasms with paroxysmal stabbing maxillary or mandibular pain
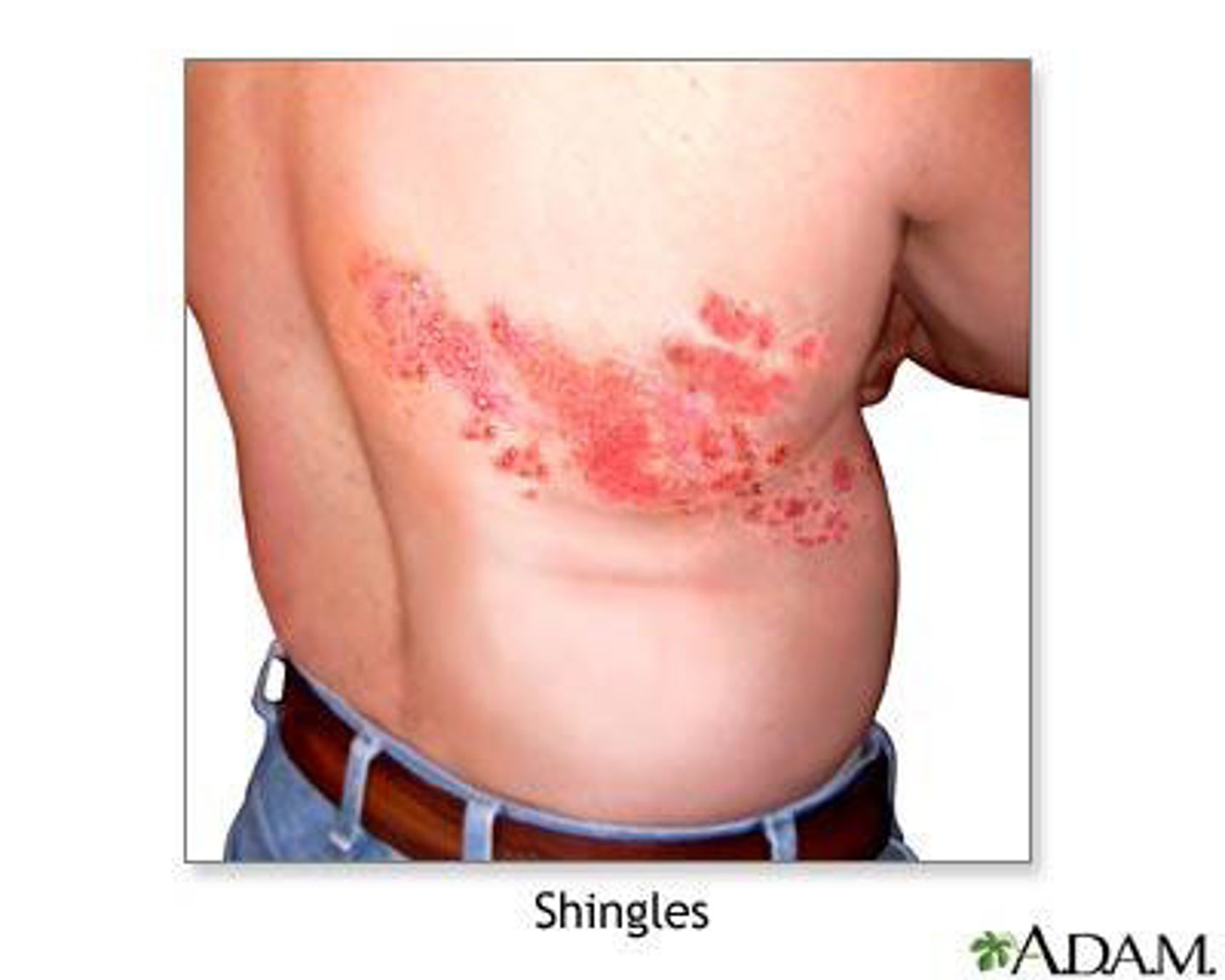
what is post-herpetic neuralgia?
neuralgia of the nerves associated with herpes lesions
- occurs along a dermatome (unilateral)
- may persist beyond the resolution of the lesions
what are the common causes of neuropathic pain?
- DM
- spinal herniation or disc issues
- vitamin deficiency (B1/6/12, E, niacin)
- stress injury (carpal tunnel)
- meds. (chemo., isoniazid)
- infections (shingles, HIV, Lyme)
- CA
- toxins (arsenic, lead, mercury)
list the 6 ways of classifying pain
- type: nociceptive, inflammatory, neuropathic
- duration: acute or chronic
- malignant vs. non-malignant
- severity: mild, moderate, severe
- etiology
- anatomical location
acute pain is usually ______________; what typically causes acute pain, and how long does it last?
nociceptive
- occurs abruptly due to surgery, trauma, labor, acute illness
- lasts <30 days
- resolves as the cause/event heals
- may become chronic if untreated
- serves a warning to prevent further harm!
describe the characteristics of chronic pain; how is it classified, and how long does it last?
persistent pain that lasts >3 months
- lasts longer than the anticipated healing process
- can be nociceptive, neuropathic/functional, or mixed
- classified as CA or non-CA
- changes nerve fibers, making it hard to treat!
is CA/malignant pain usually acute, chronic, or both? what causes this pain?
acute AND chronic; caused by...
- disease (tumor invasion, obstruction)
- procedures (biopsies, surgery)
- treatment (chemo, radiation)
list examples of CA pain syndromes
- spinal cord compression
- bone metastases
- bone marrow expansion
- plexopathies
- brain metastases
- visceral pain due to liver, pancreas, or GI tumors
- treatment-related (neuropathies, mucositis, post-op, meds)
autonomic Sx are often present with acute pain but are NOT considered _______________; provide examples
diagnostic
- tachycardia
- HTN
- diaphoresis
- mydriasis
- pallor
distress can also be indicative of acute pain!
T/F: autonomic Sx are often NOT present with chronic pain
TRUE
- patient may not appear to be suffering
- may instead present with anxiety, depression, fatigue, anger, or insomnia
what are the 4 main components of a patient assessment for pain?
- diagnostic indicators
- effectiveness of interventions
- worsening of underlying illness
- impact on functioning and QOL
when assessing a patient's pain Hx, what subjective information should you collect?
- onset
- description
- localization
- radiation
- intensity
- exacerbating/relieving factors
- impact on function and QOL
- previous pain Hx
we can use the abbreviation PQRST to remember how to assess a patient for pain; what do each of these letters stand for?
- P: palliative of provocative factors (what makes the pain better or worse?)
- Q: quality/description (ex: stabbing, burning, sharp)
- R: radiation
- S: severity
- T: temporal factors (does the pain change with time?)
what are some ways we can quantify a patient's pain?
- visual analog scales
- numeric scales (0-10, 10 being the worst pain you can IMAGINE)
- verbal descriptors (mild, moderate, severe, excruciating)
what are the factors included in a multidimensional pain assessment?
- Hx
- quality
- location
- impact on function
- relationships of pain
- relief from current interventions
what populations may require special considerations when assessing them for pain?
- infants and small children → look for irritability, crying, change in normal behaviors
- cognitive impairment → look for visual/behavioral evidence and VS
- nonverbal/language barrier → watch body language
- elderly → more likely to have pain for a long time and have since adaptive
- delirium or uncooperativeness → VS and physical or behavioral evidence
what are the consequences of uncontrolled pain?
- unnecessary suffering
- decreased QOL
- decreased activity, appetite, and sleep
- physiologic changes
- increased HC costs (disability, length of stay)
what physiologic consequences are observed due to uncontrolled pain?
- decreased healing
- impaired respiration
- impaired immune response
- increased thromboembolic complications
- increased anxiety and depression
how do exogenous pain modulators mediate peripheral pain?
- block algesic mediators
- regional analgesia
- neural ablation
how do exogenous pain modulators mediate spinal pain?
- block excitatory NTs
- enhance inhibitory NTs
how do exogenous pain modulators mediate supraspinal pain?
interfere with pain perception
what are some non-pharmacologic pain management techniques?
- cognitive-behavioral interventions → distraction, relaxation, imagery, biofeedback (deep breathing, self-soothing)
- physical agents → heat, cold
- stimulus-induced → TENS, acupuncture
- neuroablation (destroying nerves!)
what pharmacologic agents are often used to treat pain?
- NSAIDs
- corticosteroids
- acetaminophen
- opioids
- co-analgesics → antiarrhythmics, anticonvulsants, antidepressants
- local and general anasthetics