Heat Capacity
0.0(0)
0.0(0)
Card Sorting
1/5
Earn XP
Description and Tags
Flash cards for lecture 4 of thermo
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
6 Terms
1
New cards
What is heat capacity and what’s its equation.
Heat capacity, Cs, refers to the heat supplied divided by the temperature change.

2
New cards
What is he unit for heat capacity per unit mass
Heat capacity per unit mass = J K^-1 g^-1
3
New cards
What’s the symbol and units for Molar heat capacity
Molar heat capacity, Cm, has units of J mol^-1 K^-1
4
New cards
Equation for q using molar heat capacity
q = n Cm ΔT
5
New cards
What are the two values for Cm when gaseous
For gases, there are two values of Cm
Molar heat capacity at constant pressure Cp in J K^-1 mol ^-1
Molar heat capacity at constant volume Cv in J K^-1 mol ^-1
6
New cards
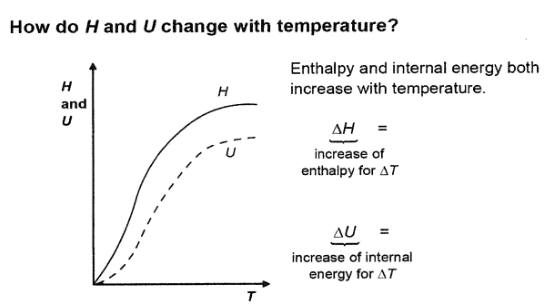
Describe the graph of how H and U change with temperature