Rates of reaction core practicals
1/9
Earn XP
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
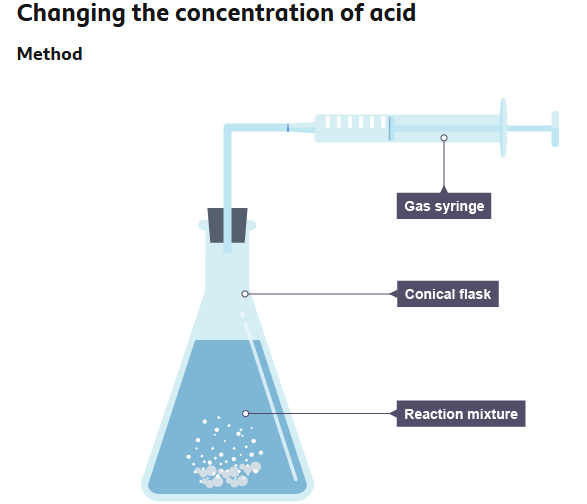
Changing the concentration of acid
methods for changing the concentration of acids
Support a gas syringe with a stand, boss and clamp.
Using a measuring cylinder, add 50 cm3 of dilute hydrochloric acid to a conical flask.
Add 0.4 g of calcium carbonate to the flask. Immediately connect the gas syringe and start a stop clock.
Record the time for every 10 cm3 of gas produced.
When the reaction is complete, clean the apparatus as directed by the teacher.
Repeat steps 1 to 5 with different concentrations of hydrochloric acid.
changing the temp
Carry out the experiment described above but:
keep the concentration of acid the same
warm the acid to different temperatures using a hot water bath, or a Bunsen burner, tripod and gauze
measure and record the temperature of the acid
describe the effect of increasing the temperature on the mean rate of reaction
Changing the surface area to volume ratio
Carry out the experiment described above but:
keep the temperature and concentration of acid the same
use different sized pieces of calcium carbonate, including a powder
describe the effect of increasing the surface area to volume ratio (decreasing the particle size) on the mean rate of reaction
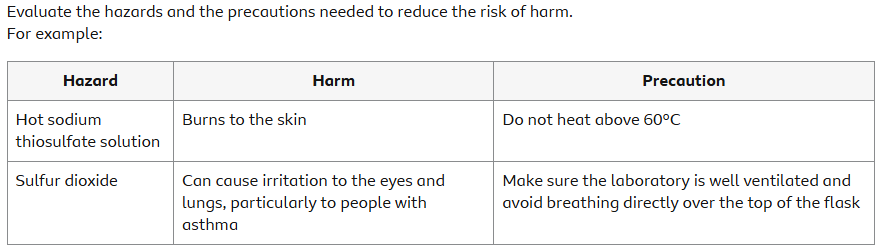
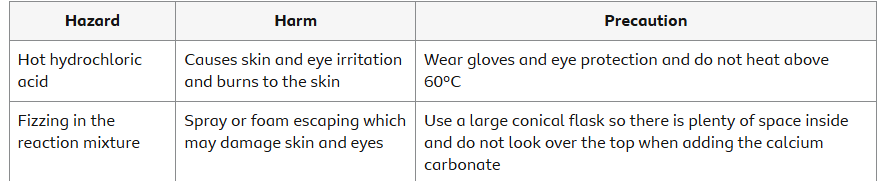
Hazards, risks and precautions
It is important in this practical activity to use appropriate apparatus and methods. This includes the safe use and careful handling of substances.
Evaluate the hazards and the precautions needed to reduce the risk of harm. For example:
observing colour changes
There are a number of ways to investigate the rate of a reaction in Chemistry. This is an outline of the required steps to undertake one of these methods. It is important in this core practical to use appropriate apparatus to make and record a range of measurements accurately, including mass, time, temperature and volume.
Aims for observing colour changes
To investigate the effect of changing the temperature on the rate of a reaction.
Sodium thiosulfate solution reacts with dilute hydrochloric acid:
Sodium thiosulfate + hydrochloric acid → sodium chloride + water + sulfur dioxide + sulfur
Na2S2O3(s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + SO2(g) + S(s)
The sulfur forms a cloudy yellow-white precipitate during the reaction. The time taken for this to achieve a given cloudiness provides a way to measure the reaction time.
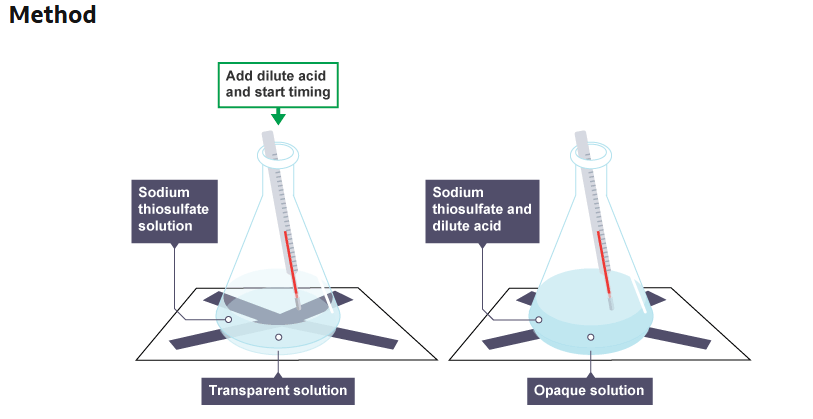
Method of observing colour changes
method of observing colour changes (this is the method non image)
Using a measuring cylinder, add 50 cm3 of dilute sodium thiosulfate solution to a conical flask.
Place the conical flask on a piece of paper with a black cross drawn on it.
Using a different measuring cylinder, add 10 cm3 of dilute hydrochloric acid to the conical flask. Immediately swirl the flask to mix its contents and start a stop clock.
Measure and record the temperature of the reaction mixture.
Look down through the reaction mixture. When the cross is no longer visible, record the time on the stop clock.
Measure and record the temperature of the reaction mixture, and clean the apparatus as directed by the teacher.
Repeat steps 1 to 6 with different starting temperatures of sodium thiosulfate solution.
Hazards, risks and precautions for observing colour changes