L8 QMOT: Building from Fragments
1/11
Earn XP
Description and Tags
Qualitative Molecular Orbital Theory
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
12 Terms
What is the first step to work out the orbital interactions of molecules of several atoms
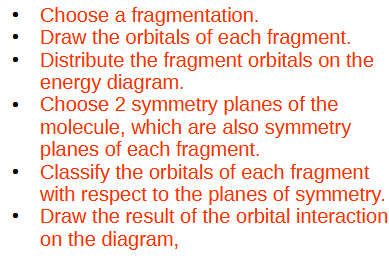
Break it into fragments. Don’t need to follow any chemical rules but ideally keep the symmetry of the molecule. A single plane ought to be a symmetry element of both fragments (in this case down the middle)

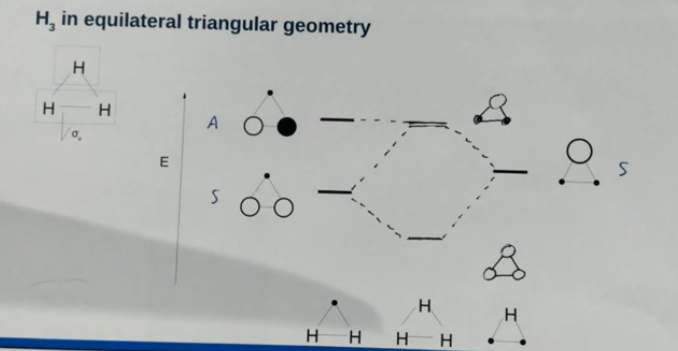
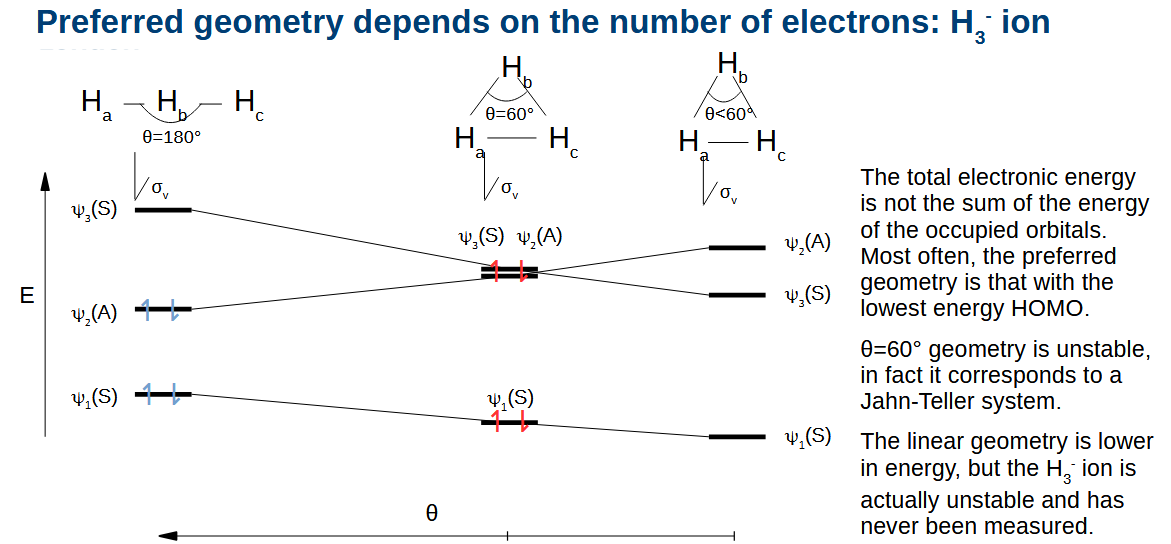
Drawing H3 triangular geometry orbitals
Draw the bonding/antibonding combinations as necessary for each fragment and determine their symmetry in the plane. Only orbitals of the same symmetry will combine. The antisymmetric orbital does not combione and for this molecule just happens to have the same energy as the out of phase combination of the other orbitals.
Drawing linear H3 orbitals list of steps
Drawing linear H3 orbitals
Make sure when listing multiple symmetries that you say which order they are in.
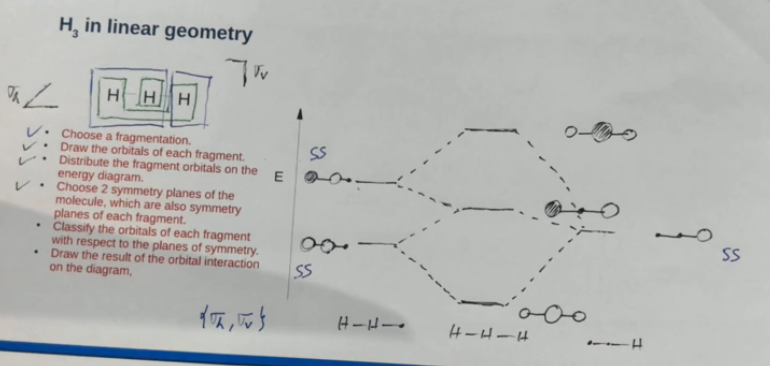
Correlating the orbitals from these different geometries
During the bending of the terminal atoms, any symmetry planes which remain lead to the same symmetry being shown from the orbitals.
The orbital energies change as you bend H3 as the bonding/antibonding interactions strengthen.
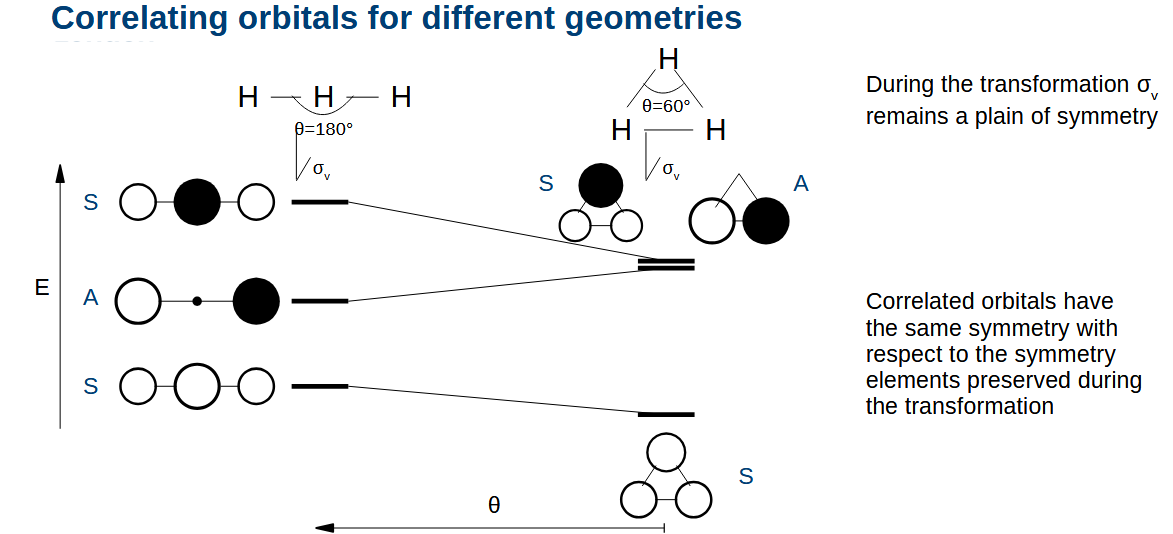
What happens when you decrease the angle further
The orbital energies change again. You can tell which orbital was which originally as their symmetries are preserved, as the symmetry cannot change without being symmetric and anti-symmetric at the same time.
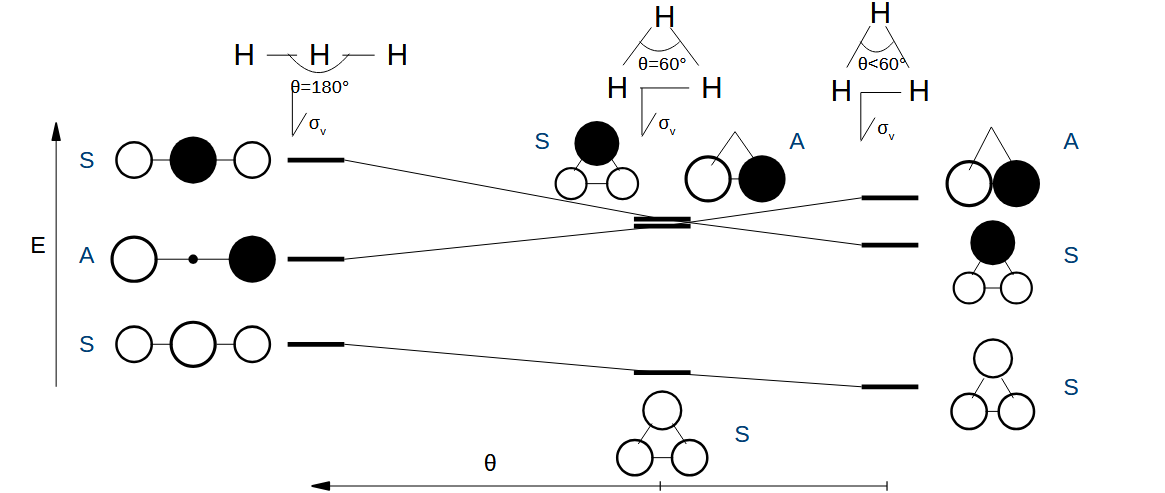
Why is the preferred geometry for H3+ not less than 60 degrees
The exchange integral HAB goes way up at low internuclear distances, the proprtionality to the overlap integral stops.
What generally determines the preferred geometry in temrs of orbaital energies
The most stable geometry is typically the one with the lowest energy HOMO. If you add more electrons to H3, the lower HOMO wins out even though the lowest energy electrons went up in energy.
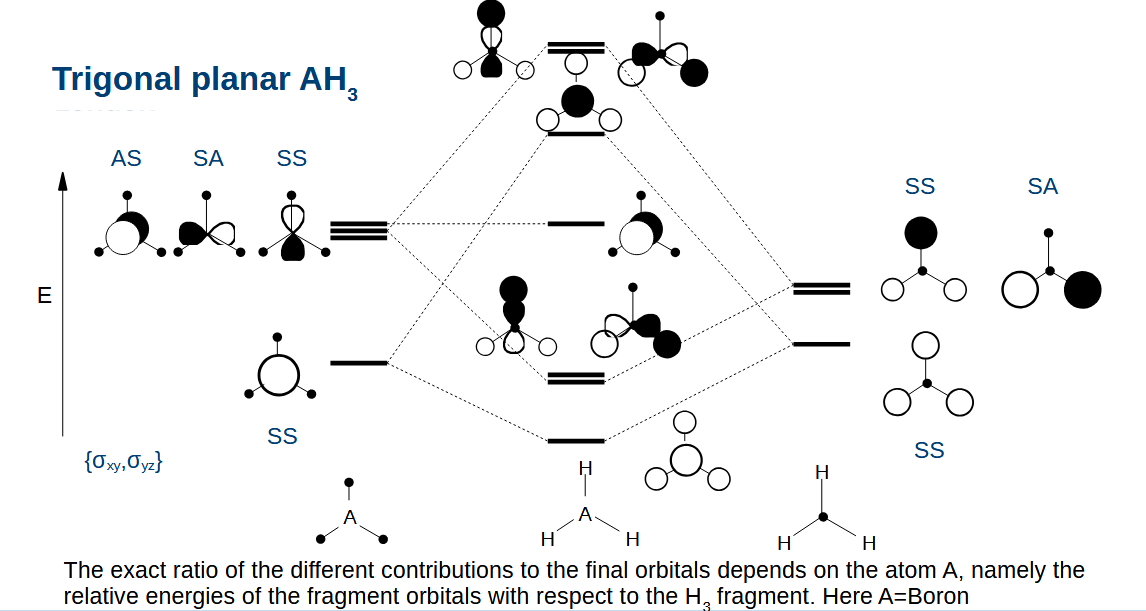
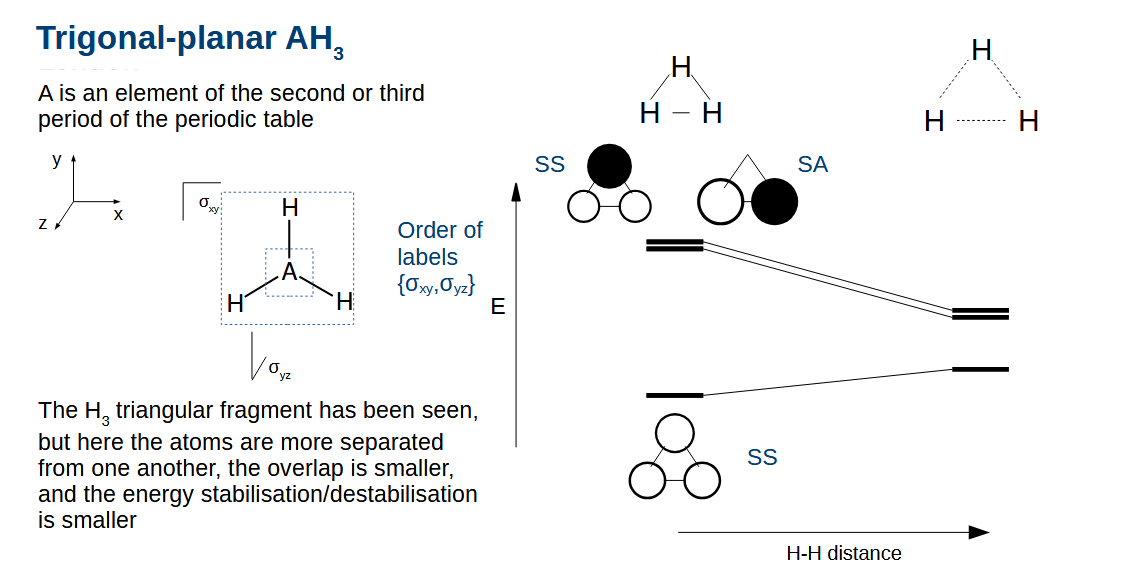
AH3 orbital diagram - how do we adapt the diagram to draw the H3 fragment
Reduce the stabilisation/destabiliastion as the orbitals are further apart.
Drawing the AH3 orbitals - why do some orbitals with the same symmetry have zero overlap
They have a different number of nodes:
Top left 1:0 bottom right
Bottom left 0:1 Top right
(nodes come about from changes in sign)
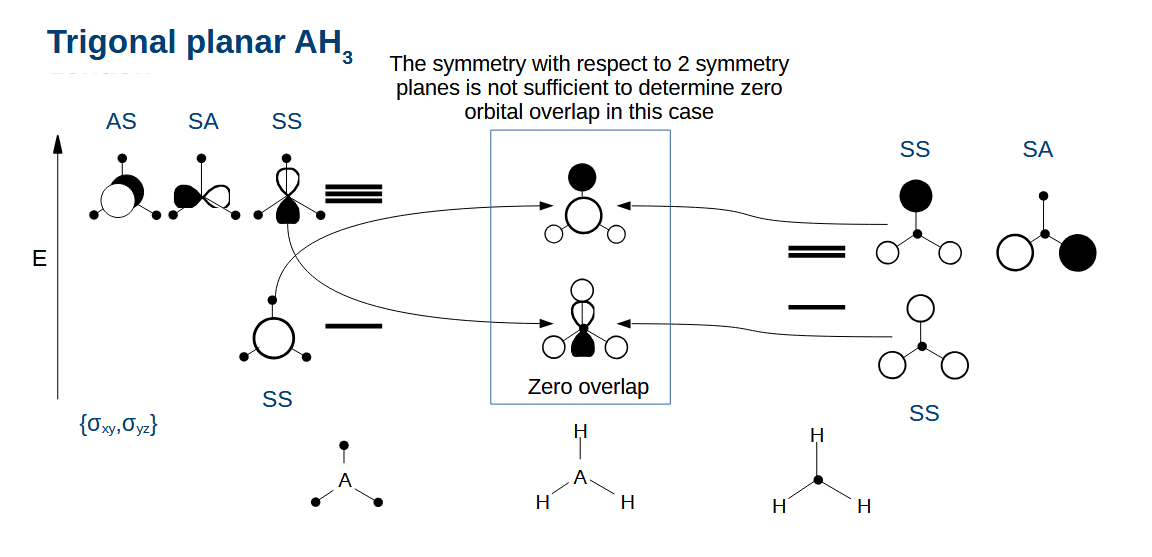
Combining the AH3 orbitals
Combine orbitals with the same symmetry as usual
Remember that the electronegativity of the atom affects the relative energy levels of the orbitals on the left.