🫁 Alveolar Gas Exchange 1
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
16 Terms
What happens to gas partial pressures in wet air?
Water vapor saturates air at the air–water interface.
Water vapor contributes to total pressure and displaces other gases.
This occurs at the air–lung interface, because the lungs have wet surfaces.
Air is humidified on its way to the lungs.
Water vapor pressure depends on temperature:
At 100°C, water vapor pressure = 760 mmHg
At 0°C, water vapor pressure = 5 mmHg
JW HY: Part of the way that we lose water is called insensible water loss, and it's water loss simply from the fact that every time we breathe, some water is leading through our airways. We're moistening it on the way in, and we're losing water on the way out.
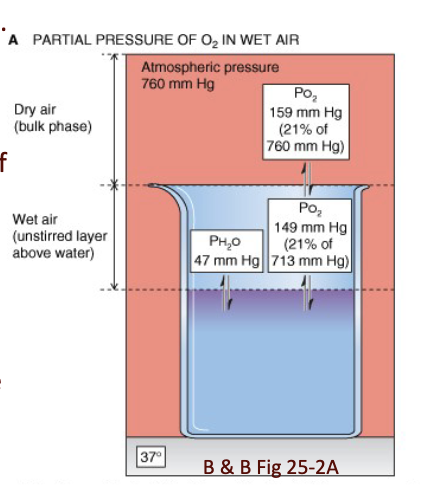
What is the partial pressure of water at 37°C, and how does this affect the partial pressures of other gases at the lung surface?
PH₂O at 37°C = 47 mmHg
At the air–lungs interface:
P_total = 760 − 47 = 713 mmHg
This reduces partial pressures of all gases at the lung surface:
PN₂ = 713 × 0.79 = 563.3
PO₂ = 713 × 0.21 = 149.7
PCO₂ = 713 × 0.0004 = 0.28
Fraction of water vapor stays the same at lung/water interface.
JW HY: Well, the air in our lungs is 20% of 713, not 20% of barometric pressure. Okay, because we are displacing some of that.
what are the alveolar and expired air partial pressures, and why do they differ?
Alveolar air:
PO₂ = 104 mmHg
PCO₂ = 40 mmHg
Alveolar air is only partially replaced by atmospheric air.
O₂ constantly diffuses from alveoli into blood.
CO₂ constantly diffuses from blood into alveoli.
Expired air:
PO₂ = 120 mmHg
PCO₂ = 27 mmHg
Expired air = alveolar air mixed with dead space atmospheric air.
O₂ concentration higher in atmospheric air than alveolar air.
CO₂ concentration lower in atmospheric air than alveolar air.
JW HY: So there was really no partial pressure in the air of CO2. And yet, I'm telling you, in our alveoli, we have a CO2 of about 40 millimeters of mercury. And so, one of the reasons for this is when we're inhaling, the air in our alveoli is only partially replaced by atmospheric air.
CO2 from metabolism ***
What does Henry’s Law state about the partial pressure of a gas in a fluid?
Henry’s Law:
At a constant temperature, the amount of a given gas dissolved in a liquid is directly proportional to the partial pressure of that gas in equilibrium with that liquid.
Formula:
P_gas (in fluid) = [gas] / solubility
JW HY: So the partial pressure of a gas in a fluid is going to be the concentration of that gas divided by the solubility of the gas essentially water, right? Because blood, the basic component is water.
![<p><strong>Henry’s Law:</strong><br>At a constant temperature, the amount of a given gas dissolved in a liquid is <strong>directly proportional</strong> to the partial pressure of that gas in equilibrium with that liquid.</p><p>Formula:</p><ul><li><p><strong>P_gas (in fluid) = [gas] / solubility</strong></p></li></ul><p>JW HY: So the partial pressure of a gas in a fluid is going to be the concentration of that gas divided by the solubility of the gas <strong>essentially water, right? Because blood, the basic component is water.</strong></p><p></p>](https://knowt-user-attachments.s3.amazonaws.com/f07203b5-b4e1-4aba-ab2b-773cb2eb9ece.png)
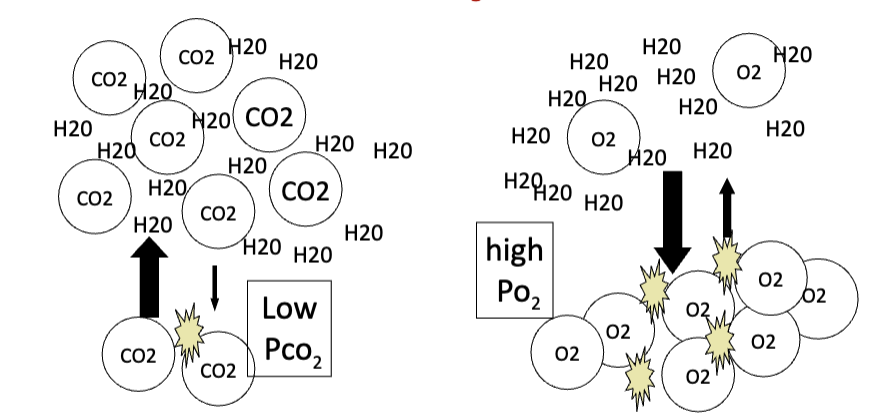
How does solubility affect the partial pressure of gases in a fluid?
If two gases are present at the same concentration, the less soluble gas displays a higher P_gas.
CO₂ is 20× more soluble than O₂.
Therefore:
Low PCO₂
High PO₂
JW HY: It's gonna have more pressure in the fluid, because it didn't dissolve, essentially.
So, the reason CO2 is in soda is because we would have to have, like, 20 times more pressure in an O2 canister, to actually carbonate that water than what we have to do for CO2. And you can see, it's hard to imagine that a gas is dissolved, but if you just think of a 2-liter bottle of soda.
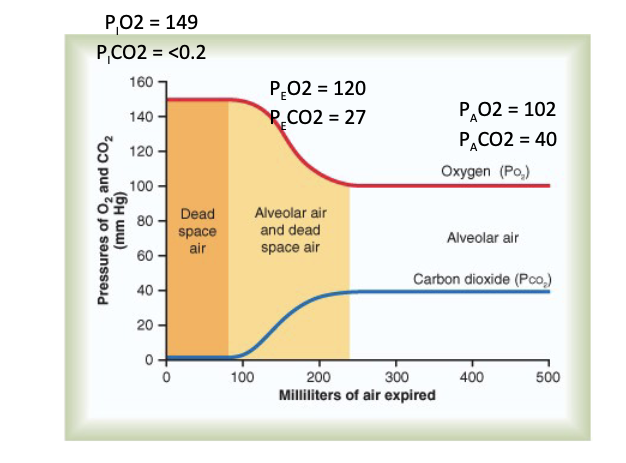
What causes the changes in PO₂ and PCO₂ as air travels to the alveoli?
Mixing of:
Atmospheric air
Dead space air
Alveolar air
Results in:
Fall in PO₂
Rise in PCO₂
by the time air reaches alveoli.
Slide values shown:
P_IO₂ = 149, P_ICO₂ < 0.2
P_EO₂ = 120, P_ECO₂ = 27
P_AO₂ = 102, P_ACO₂ = 40
JW Hy:
Like, you're trying to really get them to get as much air out as they can, and that's what it will take to get these numbers out here ie alveolar air. So, under normal inhalation, exhalation, we're going to see these values ie dead space air and alveolar air + dead space
What is minute ventilation and how is it calculated according to the slide?
Minute ventilation = total air volume exchanged (inhaled or exhaled) per minute.
V_E = V_T × f
Example given:500 mL/breath × 15 breaths/min = 7500 mL/min
(for average 70 kg male)
JW HY: Now, alveolar ventilation is different because it is the amount of air exchanged by the alveoli per minute, and that's a different number because Not every breath, of course. I already said a whole bunch of air gets trapped in this dead space, so not every breath, all of the air is going to the alveoli. —> anatomical deadspace
How is alveolar ventilation defined and how does dead space affect it?
Alveolar ventilation = total air volume exchanged by the alveoli per minute.
Different from V_E because only part of each breath reaches alveoli.
Anatomic dead space (V_D):
Air in non–gas-exchange airways.
Usually constant, but varies with lung volume and posture.
How does the slide estimate anatomic dead space and calculate alveolar ventilation?
Estimate:
~1 mL V_D per lb body weight
150 lb male → 150 mL dead space
Calculation:
V_A = (V_T − V_D) × f
Example:
(500 − 150) ml/breahte× 15 = 5250 mL/min
JW HY: Now, coincidentally, this number is very close to what is normal cardiac output. 5 liters per minute,—> moving cardiac output across those surfaces, because the right heart is doing the same thing as the left heart. So we have a pretty good match here of air being moved and blood being moved across the surfaces.
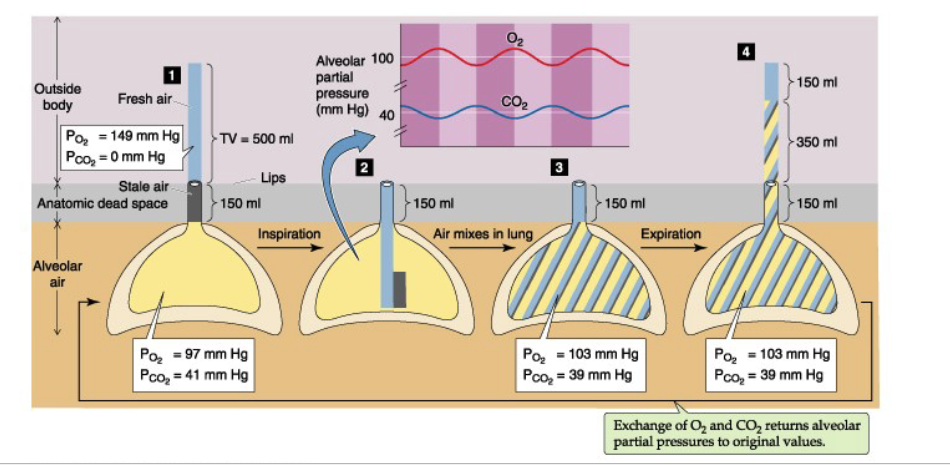
What does the slide illustrate about how rapid/shallow vs slow/deep breathing affects alveolar ventilation?
Pre-surgery:
V_T = 500 mL
f = 15
V_E = 7500 mL/min
V_A = (500 − 150) × 15 = 5250 mL/min
Post-surgery:
V_T = 200 mL
f = 40
V_E = 8000 mL/min
V_A = (200 − 150) × 40 = 2000 mL/min
Conclusion:
Rapid & shallow breathing → reduces V_A
Slow & deep breathing → enhances V_A
JW HY: Because the breadths are so shallow, you'll see here, if I take that tidal volume of 200 minus the dead space volume, which didn't change for this person.
Only 50 mils per breath is actually getting in for gas exchange.
Which means that now we're only at 2,000 or 2 liters per minute of actual gas exchange at the alveoli.
What is physiologic dead space according to the slide?
Physiologic dead space = non-airway dead space that occurs in alveolar regions.
In healthy young individuals: almost all alveoli are ventilated and perfused.
Disease (e.g., pulmonary embolus) can block blood flow.
Ventilated but not perfused alveoli → no gas exchange → increases physiologic dead space.
JW HY: So even though the person's breathing may look normal.
But if a portion of the lungs that are not participating, and there's no gas exchange, then we say that that would reduce alveolar ventilation
What determines the alveolar PO₂ ?
Normal steady-state P_AO₂ = 102 mmHg.
P_AO₂ depends on:
O₂ entering, determined by:
Inspired oxygen (P_IO₂)
Alveolar ventilation (V_A)
P_AO₂ is directly proportional to P_IO₂ and V_A
JW HY: So, the inspired amount of O2 is determined by where you are in the environment. So we always use sea level, we always say 760 times 0.21, but when the environment changes,
What does the slide show about the alveolar gas equation?
The alveolar gas equation is shown.
R = respiratory gas quotient (VCO₂/VO₂)
If only carbohydrate is used:
R = 1
If fat is primary fuel:
R = 0.7
Under normal circumstances:
R = 0.8 (So that means that more oxygen is used than the amount of CO2 that's released in the alveoli.)
JW HY: R = one CO2 for each O2 consumed, based on this perfect oxidative metabolism of glucose.
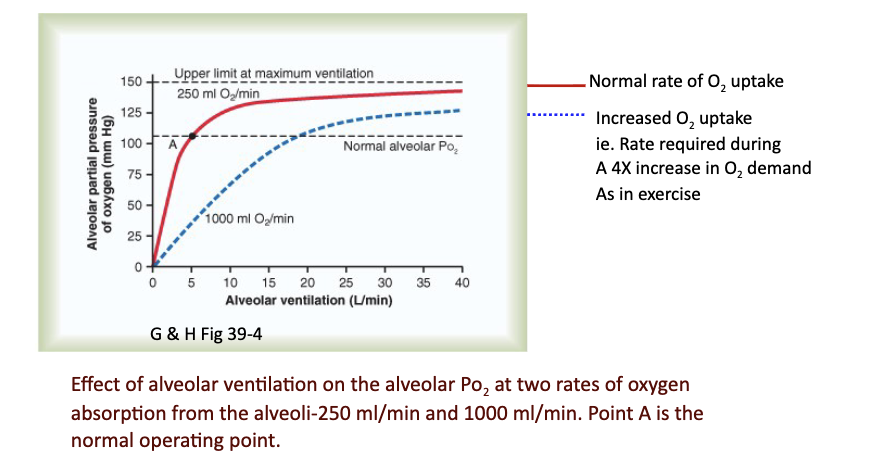
What determines O₂ concentration and PO₂ in the alveolus?
It depends on the balance between:
O₂ diffusion into blood
New O₂ entering due to ventilation
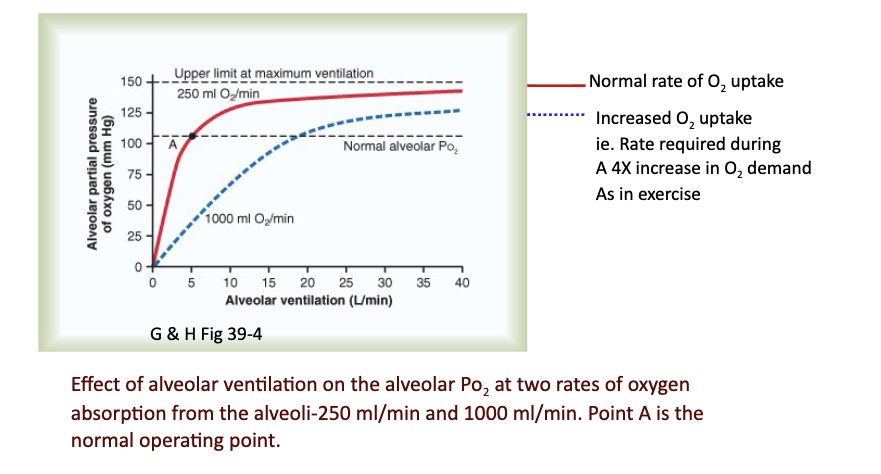
Slide includes graph of effect of ventilation and different O₂ absorption rates (250 vs 1000 mL/min).
JW Hy: Red line is that if I decrease alveolar ventilation to 4 liters per minute—> fall in my partial pressure, because I'm not bringing in as much air as I need to support normal resting O2 uptake.
If I ventilate more than what I need, —> not changing my metabolism, —> then increase the partial pressure of O2 in the lungs.
What determines alveolar PCO₂ according to the slide?
Normal steady-state P_ACO₂ = 40 mmHg
P_ACO₂ = CO₂ entering − CO₂ leaving
CO₂ entering is determined by tissue CO₂ production (VCO₂)
Atmospheric CO₂ ≈ zero
Rate of CO₂ leaving lungs = alveolar ventilation (V_A)
Therefore P_ACO₂ is:
Directly proportional to CO₂ production
Inversely proportional to V_A
JW HY:
the partial pressure of O2 is directly proportional to how well we breathe in towards our alveoli.
Partial pressure of CO2 is inversely proportional. The more that I breathe, the more CO2 I blow off.
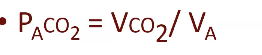
What does the slide illustrate about the effect of alveolar ventilation on alveolar PCO₂?
The graph shows:
Alveolar PCO₂ depends on alveolar ventilation.
Two CO₂ excretion rates shown: 800 mL/min and 200 mL/min.
The normal operating point is labeled Point A.