exam #1: aquaporins, insulin, carbonic anhydrase
1/43
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
44 Terms
when proteins don’t work ___, typically occurs
disease
what parts of the membrane are polar / nonpolar?
polar phospholipid head (exterior), non-polar phospholipid tails (interior)
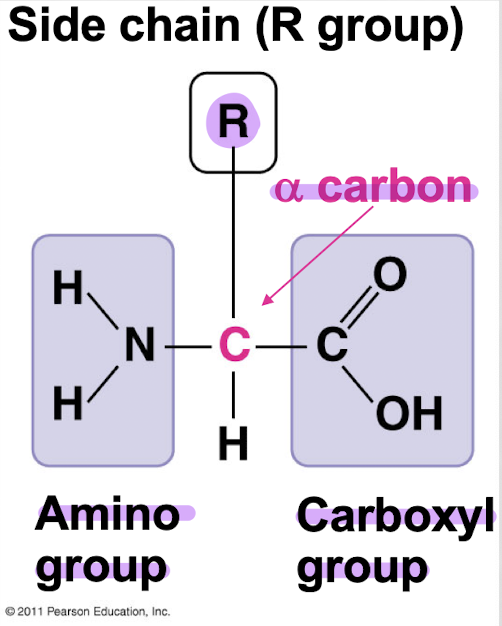
what are the 3 components of an amino acid?
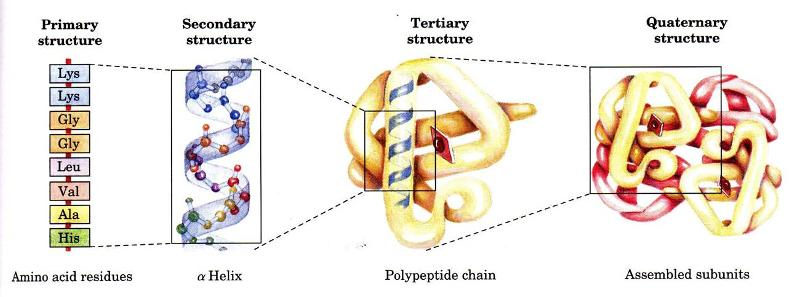
what are the different levels of protein structure?
primary structure: consists of amino acids connecting through peptide bonds
secondary structure: consists of the amino acid backbone bonding through hydrogen bonds
tertiary structure: consists of R groups (side chains) that bond together and fold into a 3D structure. This level consists of several possible bonds, including H bonds, disulfide bonds, ionic bonds, and van der Waals forces
quaternary structure: consists of multiple tertiary structures bonding together with one another. This level consists of the same interactions as the tertiary level
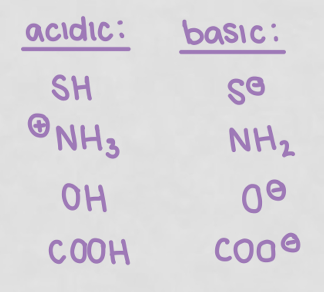
how to determine if an amino acid is polar or nonpolar?
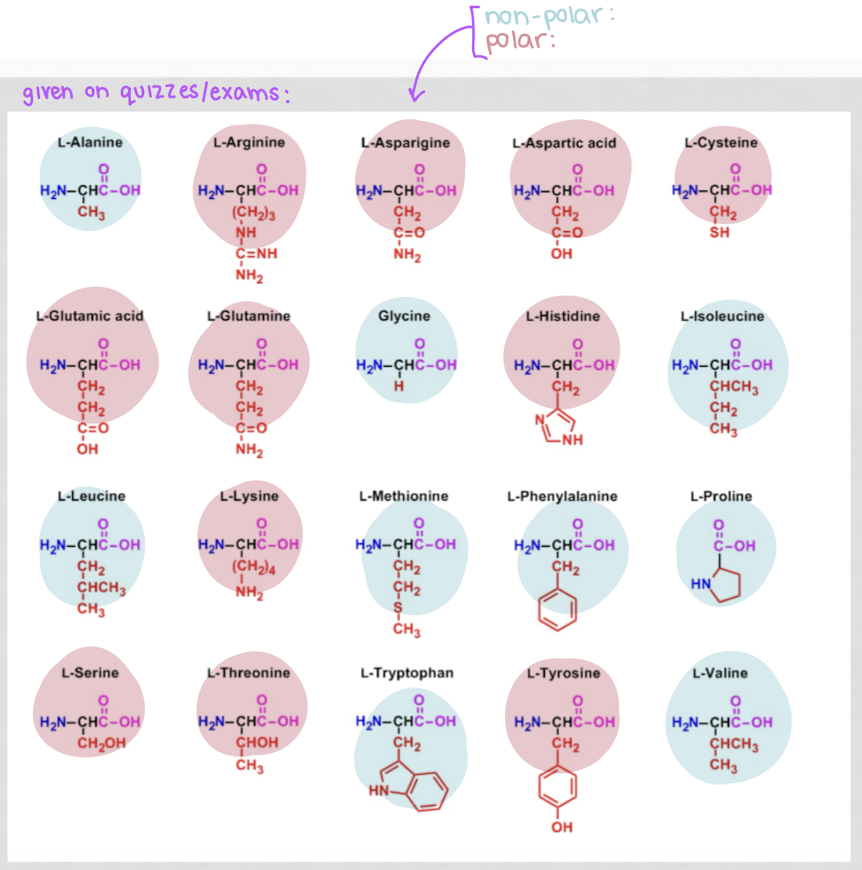
with the side chain, its polar if it:
has an oxygen
has more than 1 electronegative atom in the R group
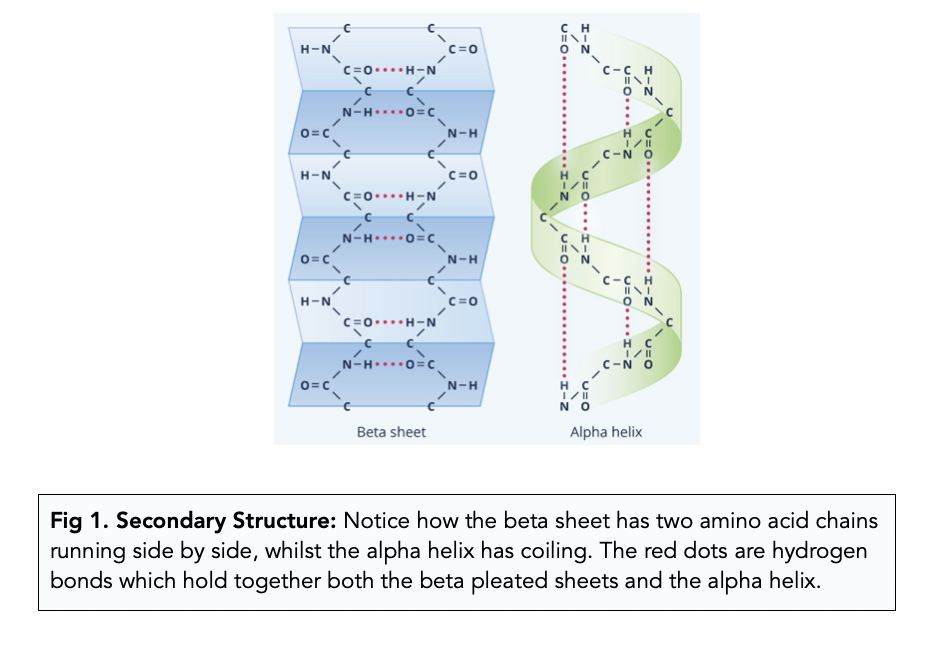
how does hydrogen bonding give rise to an alpha helical structure? how would changes in amino acids potentially impact this hydrogen bonding?
the alpha helix is stabilized by hydrogen bonds between the carbonyl oxygen (C=O) of one peptide bond and the amide hydrogen (N-H)
changing amino acids can affect the structural interaction based on the charge, bonds between R groups, and steric hindrance based on size
what are the different types of view models?
space-filling model, ribbon model, ball-and-stick model
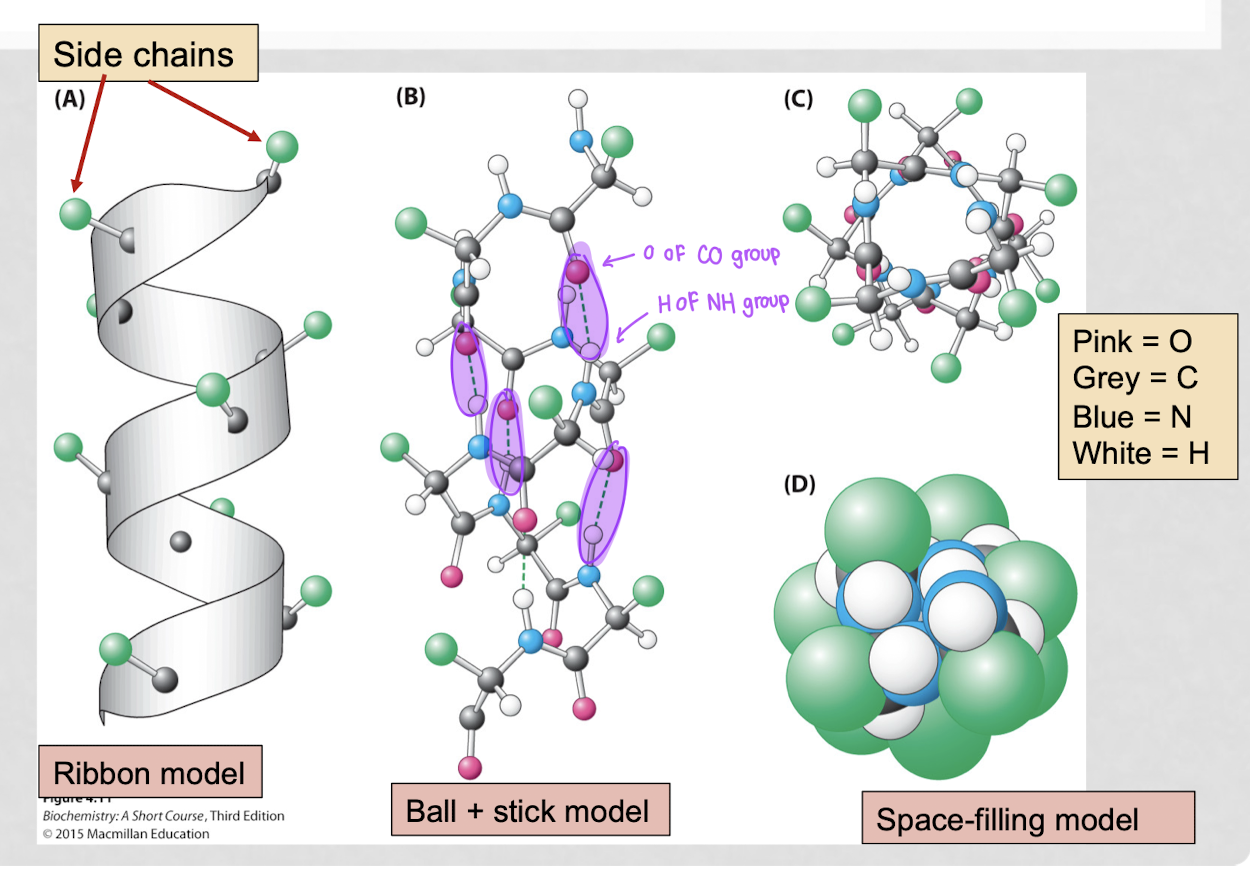
what is the space-filling model?
represents atoms as spheres scaled to their van der Waals radii
best for: visualizing molecular shape, size, steric hindrance, and intermolecular interactions
what is the ribbon model?
depicts the structural backbone of macromolecules (proteins, DNA)
best for: understanding secondary/tertiary structures, folding patterns, and functional regions
what is the ball and stick model?
shows atoms as balls and bonds as sticks
best for: analyzing molecular geometry, bond angles, bonding, and functional groups
do alpha helices have a pore inside?
no, alpha helices do not have an internal pore
they are tightly coiled and stabilized by hydrogen bonds between parts of the backbone
why does there appear to be a hole in the ribbon model of an alpha helix but not in the space-filling model?
the ribbon model condenses the backbone of the alpha helix, focusing more on the general structure and the R groups of the helix rather than the accurate space taken up
the space-filling model, however, shows the full electron clouds of the atoms involved and accounts for how these clouds may take up space in the center of the helical structure
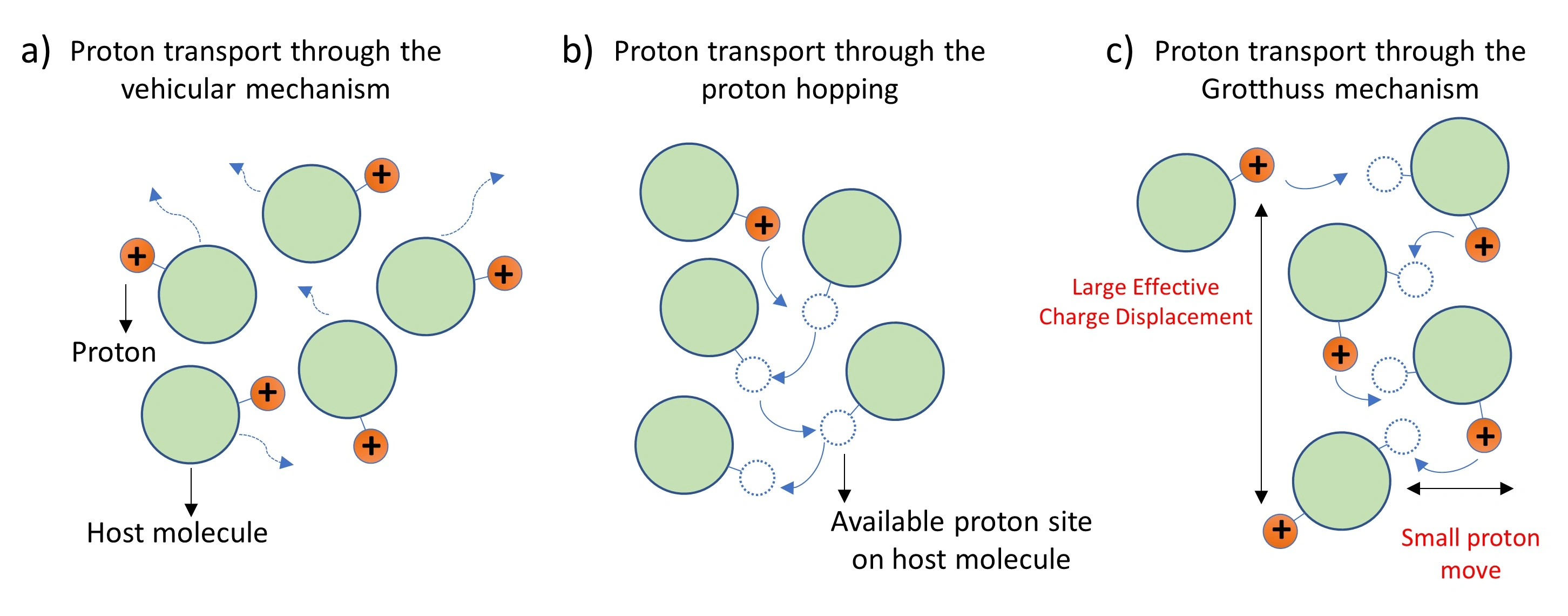
what is the grotthuss mechanism?
explains how protons (H⁺) are transferred through water or other hydrogen-bonded networks. instead of moving as individual protons, the transfer happens through a relay process:
a proton hops from one water molecule to another, forming a hydronium ion (H₃O⁺).
this causes a chain reaction where protons are passed along the hydrogen bond network.
water molecules continuously exchange roles as proton donors and acceptors.
is why protons diffuse faster in water than other ions
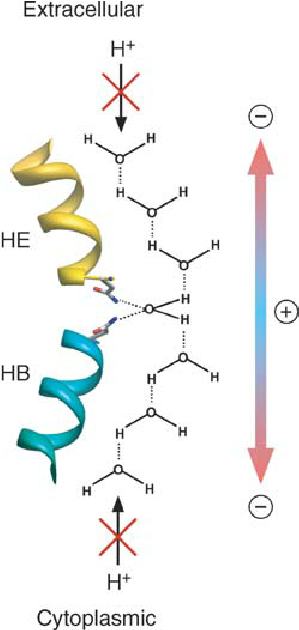
what is the NPA motif?
the NPA motif in aquaporins blocks proton transport by disrupting the grotthuss mechanism, which normally allows protons to "hop" between water molecules via hydrogen bonds
narrowing the channel – forces water into a single-file line
breaking hydrogen bonds – near the NPA motif, the water molecules rotate due to the electrostatic environment created by asparagine (N) residues. this reorientation breaks the continuous hydrogen bond network, stopping proton hopping
electrostatic barrier – asparagine (N) residues create a dipole field that repels protons
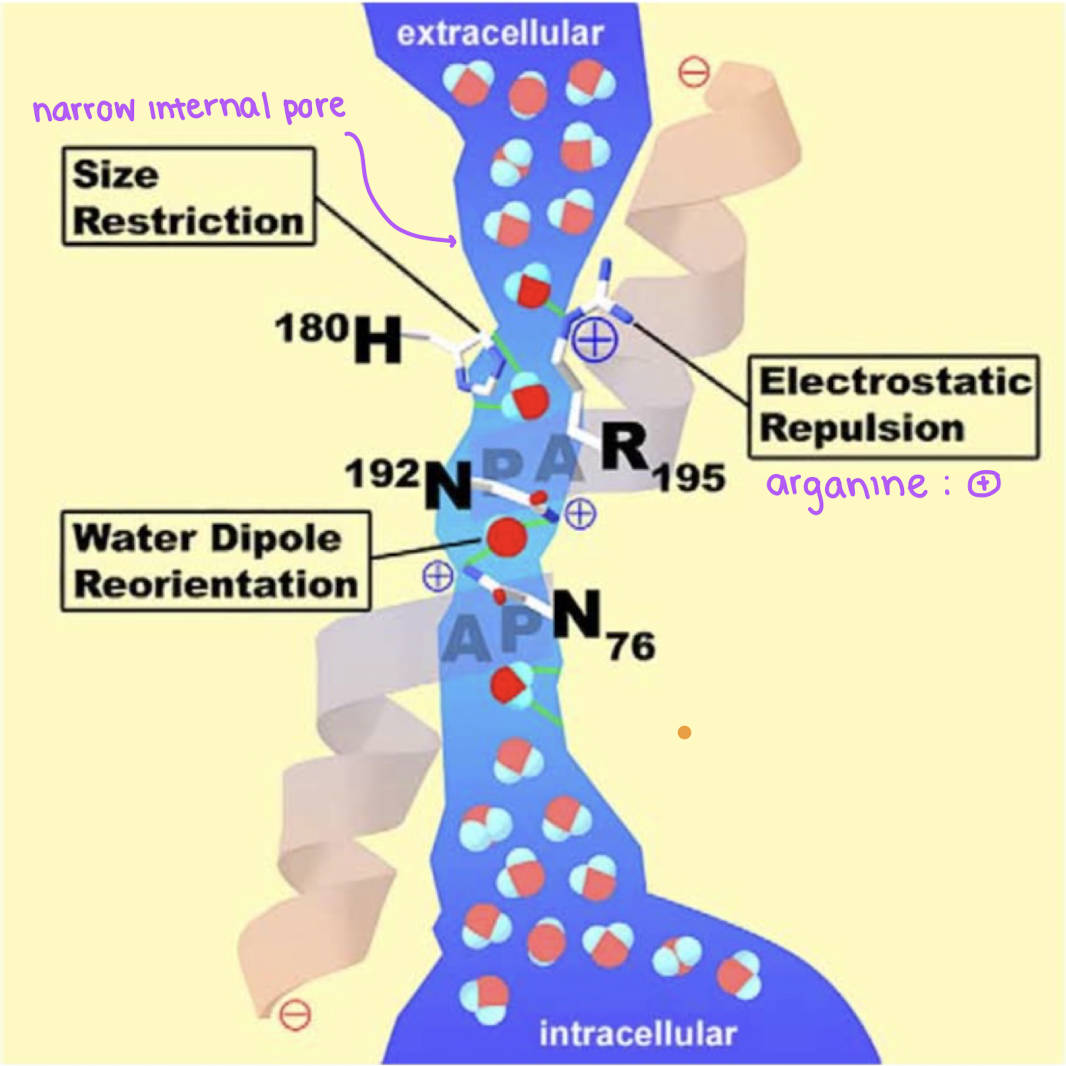
how does an aquaporin maintain selectivity for water? (3)
size restriction: the narrow internal portal going into the intracellular space is small and restricts molecules of a larger size from entering
electrostatic repulsion: arginine groups and other positively charged groups are present on the ends of the channel are positive and deter away other positively charged molecules. negative groups are present in the middle of the channel and deters negatively charged molecules from passing through
NPA motif: by asparagine disrupting the grotthus mechanism and reorienting water to break water-water hydrogen bonds, this prevents proton transfer in order to protext the pH, voltage, and other internal cellular functions
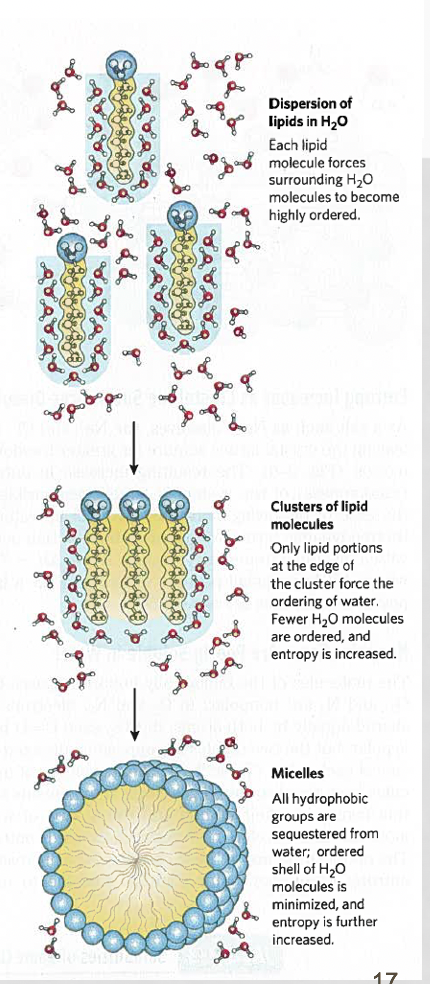
what is the hydrophobic effect?
the tendency of nonpolar substances to aggregate in aqueous solution, minimizing their exposure to water. this phenomenon drives the formation of cellular membranes and protein folding
why do micelles form?
gain of entropy of water
given that phospholipid tails are nonpolar and thus hydrophobic to water, water molecules order themselves around the tails, decreasing the entropy of the system due to water's limited movement to maintain this hydrogen bonding
the phospholipid tails do not like to interact with water and are attracted to one another through van der Waal's forces, causing them to stabilize in a sphere-like shape through these interactions
by allowing the phospholipid tails to cluster together in a micelle, this minimizes their disruption of water's hydrogen bonding and increases the entropy of the system, making it more favorable
how do non covalent interactions contribute to protein folding and stability?
hydrogen bonds: stabilize secondary structures
ionic interactions: form salt bridges between charged side chains
van der waals forces: pack atoms tightly in the protein core
hydrophobic interactions: drive nonpolar residues inward, shielding them from water
are the following full/partial charges and permanent or temporary?: ion-ion, hydrogen bond, van der waals
ion-ion: full, permanent
HB: partial, permanent
VDW: partial, temporary
why is protein folding spontaneous?
protein folding is spontaneous because it decreases the system's free energy (ΔG). this occurs through:
decrease in enthalpy (ΔH): formation of stabilizing non-covalent interactions (hydrogen bonds, ionic interactions, van der Waals forces) releases energy
increase in entropy of water: the ordered water molecules surrounding the unfolded protein become more disordered once the hydrophobic regions are buried inside the folded protein
together, these factors make the overall ΔG negative, driving the folding process
what is the pKa?
the pH at which the acid is half-dissociatedin solution, meaning that the concentrations of the protonated and deprotonated forms are equal
how do you make a polypeptide?
take the OH from the carboxy terminal and an H from the amino terminal and make H2O to form a peptide bond
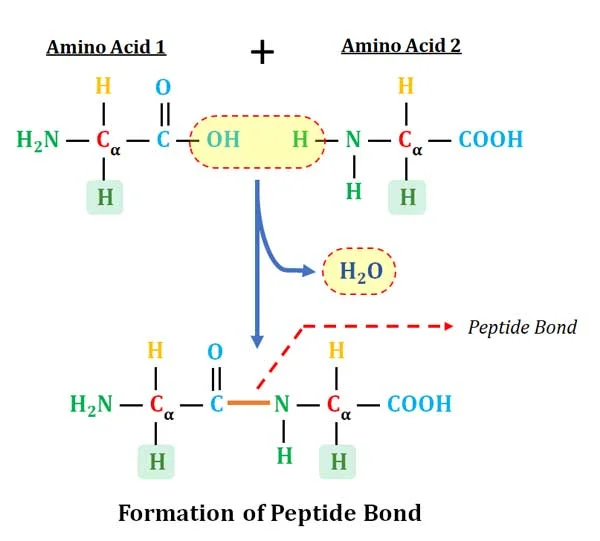
what are some characteristics of the peptide bond?
essentially planar (flexible but conformationally restricted)
the peptide bond has partial double bond character because of resonance, and thus rotation about the bond is prohibited
the bond is uncharged
when to protonate / deprotonate?
protonate: pH < pKa
deprotonate: pH > pKa
how can you change the ∆G of a reaction?
changing the concentrations
do enzymes change the ∆G?
no, but they can change the rate of the reaction
what are osteoclasts?
they break down and remove bone tissue by dissolving the fibers and the matrix of bone
when is a reaction spontaneous?
when the ∆G is negative
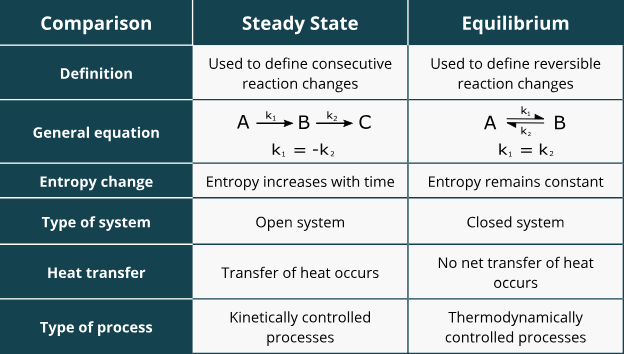
what does it mean to be at equilibrium?
the ∆G is = 0
concentrations are consistent, but the rate of reaction has not stopped its just balanced on both sides
what does ∆G tell you about the rate of reaction?
it provides no information on the rate of reaction—∆G is independent of path or molecular mechanism, only energy difference between substrate and product
what is ∆G°?
the free energy under standard conditions and at equilibrium
conditions:
pH = 7
298 K
1M reactants
what is Keq?
the equilibrium constant at standard conditions
[products]/[reactants]
Keq = 1 → ∆G° = 0 (at equilibrium)
Keq > 1 → - ∆G° (more products than reactants- spontaneous)
Keq < 1 → + ∆G° (more reactants than products - nonspontaneous)
what are the 2 different models of substrate binding and how do they differ?
the lock and key model suggests that the enzyme and substrate fit together perfectly, like a key in a lock
the induced fit model proposes that the enzyme's shape changes slightly to accommodate the substrate, creating a better fit
do enzymes change the thermodynamics of a reaction?
no, enzymes do not change the thermodynamics of a reaction; they only change the rate at which the reaction reaches equilibrium
what is Q?
the reaction quotient at non-standard conditions
[products] / [reactants]
how can the active site lower an activation energy barrier?
orientating substrates correctly
straining substrate bonds
providing a favorable microenvironment
non-covalently bonding to the substrate
where does the energy to lower the activation energy come from?
weak interactions between the enzyme and substrate, energy released when bonds form
what is a steady state?
the homeostatic condition in which net concentrations remain constant over time, thus requiring the constant input of energy from the environment (can be forward OR reversed)
how is ∆G°’ different from ∆G?
∆G°’ is the free energy at standard conditions
the concentration of the reactants and products is what determines if these two values are the same
how do the molecules in an osteoclast function with one another in the cell?
CO2 is produced by the osteoclast
CA converts CO2 + H20 to H2CO3 which readily dissociates to HCO3- and H+
the HCO3- is removed from the osteoclast by an anion exchanger, in exchange for a Cl- ion
H+ is moved actively, by a channel that uses ATP, to the resorptive pit
by pumping H+ into the resorptive pit, the low pH of 4 of the resorptive pit is achieved
Cl- is transported into the pit to keep it electrically neutral
how would a deficiency in carbonic anhydrase impact osteoclast function?
H+ would not be produced (or pumped into the resorptive pit)
thus, the resorptive pit could not be acidified and bone could not be broken down
how do enzymes like carbonic anhydrase affect the free energy of the reaction?
the overall free energy of a reaction is unaffected by an enzyme
enzymes accelerate the attainment of equilibria, but they don't shift its position
the equilibrium position is a function only of the free energy difference between reactants and products
enzymes decrease the amount of free energy that must be invested to reach the transition state
as a result, enzymes increase the rate of reactions relative to uncatalyzed reactions
why does the reaction proceed towards the formation of H2CO3 in the osteoclast even though this is not favorable under standard conditions?
the reaction proceeds toward H2CO3 because of the continuous production of CO2 by cellular processes, bicarbonate ion is being transported out of the osteoclast, and H+ is being pumped into the resorptive pit
these conditions push the reaction toward H2CO3. thus, the osteoclast is not at standard conditions of DG°'
are reactions always under standard conditions in cells?
no
concentrations of products and reactants vary, particularly in the cell where the product of one reaction is often the substrate for another