AH Chemistry | U2: Physical Chemistry | Chemical Equilibrium
1/64
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
65 Terms
Equilibrium
A chemical reaction is in equilibrium when the composition of the reactants and products remains constant indefinitely and the rates of the forward and reverse reactions are equal.
The equilibrium constant (K) characterises...
the equilibrium composition of the reaction mixture.
The value of an equilibrium constant indicates…
The position of equilibrium
The concentrations of pure solids and pure liquids at equilibrium are taken as ______ and given a value of _______ in the equilibrium expression.
Constant, 1
The numerical value of the equilibrium constant {depends on/is independent} of:
A) The reaction temperature
B) Pressure
C) Concentration
A) Dependent, as the ratio of [products]/[reactants] will be affected when the system favours either the forward or reverse reaction to increase or decrease the temperature
B) Independent of, as the system will simply temporarily favour the forward or reverse reaction to reestablish the equilibrium constant
C) Independent of, as the system will simply temporarily favour the forward or reverse reaction to reestablish the equilibrium constant
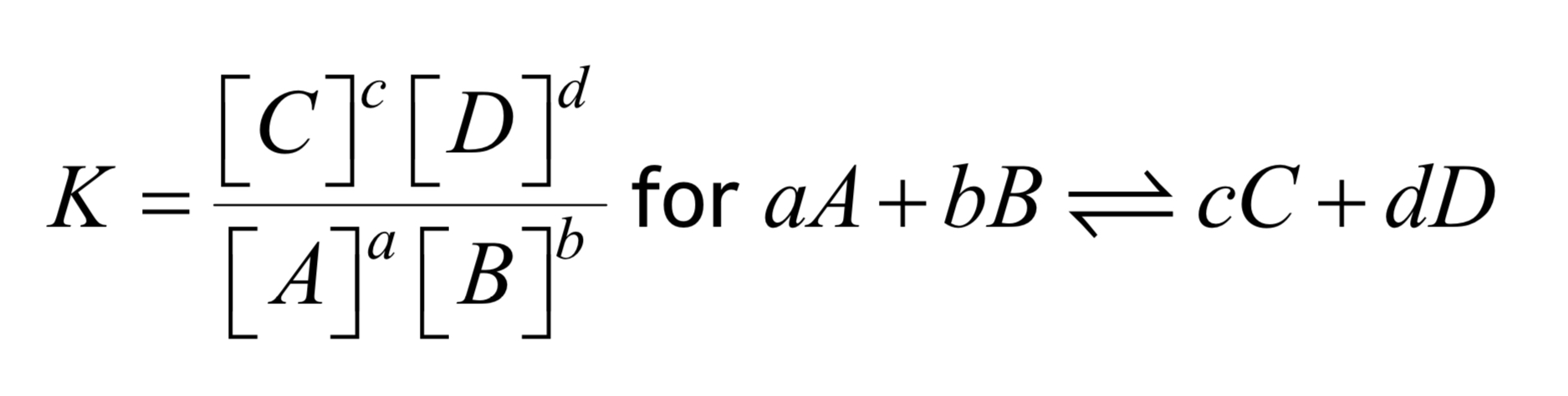
Used to calculate the equilibrium constant of a reaction. [] means concentration. For reactions involving gases p(A)a may be used instead.
When K>>1 , the equilibrium lies...
To the right (i.e. products)
When K<<1 , the equilibrium lies...
To the left (i.e. reactants)
When K is approximately equal to1, the equilibrium lies...
To neither the left nor the right
For endothermic reactions, a rise in temperature causes an {increase/decrease} in K and the yield of the product is {increased/decreased}.
For endothermic reactions, a rise in temperature causes an increase in K and the yield of the product is increased.
For exothermic reactions, a rise in temperature causes a {increase/decrease} in K and the yield of the product is {increased/decreased}.
For exothermic reactions, a rise in temperature causes a decrease in K and the yield of the product is decreased.
Effect of catalyst on equilibrium constant
The presence of a catalyst does not affect the value of the equilibrium constant.
Equilibrium of water
In water and aqueous solutions there is an equilibrium between the water molecules and hydronium (hydrogen) and hydroxide ions.
This ionisation of water can be represented by:
H₂O + H₂O ⇌ H₃O⁺ + OH⁻
H₃O⁺
hydronium ion, i.e. a hydrated proton H+(aq) + H2O (aq) -> H3O+(aq). H+ is too small to exist on its own.
A shorthand representation of H3O+ is:
H+(aq)
Water can react as an acid and as a base. State the term used to describe this.
Amphoteric
The dissociation constant for the ionisation of water, i.e. the ionic product.
The value of the ionic product {varies/is constant} with temperature as...
The value of the ionic product varies with temperature as the dissociation of water is an endothermic reaction.
The value of the ionic product is _______ at a given temperature, it is _________ by the relative concentrations of OH- and H3O+ ions.
Constant, unaffected
True or False: Neutral solutions always have a pH of 7, regardless of temperature
This pH of 7 for neutral solutions only applies at 25°C. The pH will decrease as temperature increases due to the fact that the ionisation of water is an endothermic process, therefore the value of Kw will increase with increasing temperature. Note that pOH=pH for pure water, so the solution does not get more acidic.
Concentration of H3O+ and OH- at 25oC
Both 10-7 mol l-1
pH = -log10[H3O+]
Used to calculate the pH from the hydrogen ion concentration. Taking the exponential function of both sides gives [H3O+]=10-pH .
pOH = -log10[OH-]
Used to calculate the pOH from the hydroxide ion concentration. Taking the exponential function of both sides gives [OH-]=10-pOH .
Calculating the concentration of H3O+ or OH- when one is known
Use pH + pOH = 14 or Kw
Brønsted-Lowry definitions of acids and bases
An acid is a proton donor and a base is a proton acceptor
For every acid there is a _________, formed by _________
For every acid there is a conjugate base, formed by the loss of a proton.
Conjugate base
Species formed by the loss of a proton
For every base there is a _________, formed by ________
For every base there is a conjugate acid, formed by the gain of a proton.
Strong acids and strong bases are:
completely dissociated into ions in aqueous solution.
Weak acids and weak bases are:
only partially dissociated into ions in aqueous solution.
Formula to calculate the acid dissociation constant (hint: look at indicator)
Used to calculate the approximate pH of a weak acid, where pKa is the acid dissociation constant (as seen on page 14 dta book) and c is the concentration.
Examples of strong acids include:
hydrochloric acid, sulfuric acid and nitric acid
For strong monoprotic acids (acids with only one hydrogen ion in their formula, e.g. HCl), the hydrogen ion concentration will be...
the same as the original concentration of the acid as all the acid molecules have dissociated into ions.
For strong diprotic acids (acids with two hydrogen ions in their formula, e.g. H2SO4), the hydrogen ion concentration will be...
double that of the original acid concentration since all the hydrogen ions will be released in solution.
Examples of weak acids include:
Ethanoic acid, carbonic acid and sulfurous acid
Give an example of a strong base
Metal hydroxides
Give an example of a weak base
Ammonia and amine
Explain the weakly acidic nature of solutions of carboxylic acids, sulfur dioxide and carbon dioxide with reference to equations showing the equilibria.
Explain the weakly alkaline nature of solutions of ammonia and amines with reference to equations showing the equilibria.
Equimolar solutions of weak and strong acids (or bases) have _______ pH values. Explain why.
Equimolar solutions of weak and strong acids (or bases) have different pH values, i.e. a weak acid will have a greater pH than a strong acid. This is because a strong acid is fully dissociated into its ions whereas a weak acid is only partially dissociated into its ions, so a strong acid contains a greater concentration of hydronium ions than a weak acid of the same concentration.
Equimolar solutions of weak and strong acids (or bases) have _______ conductivities and reaction rates.
Equimolar solutions of weak and strong acids (or bases) have different conductivities. This is because a strong acid is fully dissociated into its ions whereas a weak acid is only partially dissociated into its ions, so a strong acid dissolved in solution contains more ions which are able to carry a charge or react with other particles, leading to greater conductivity and faster reaction rates.
50cm3 of a strong alkali is required to neutralise 50cm3 of a strong acid. State whether a greater, lesser, or the same volume of the strong alkali would be required to neutralise the concentration of a weak acid. Explain why.
The same volume of the alkali would be required because the stoichiometry of reactions is the same, regardless of whether a strong or weak acid is used.
The hydroxide ions in the alkali react with all of the available hydrogen ions in solution. However, in a weak acid, this removes hydrogen ions from the equilibrium and causes the acid molecules to release more hydrogen ions.
This continues until all the acid molecules have dissociated, i.e. until the acid is neutralised.
The volume of alkali required therefore depends only on the concentration of the acid and not on the strength of the acid.
The acid dissociation constant
The greater the numerical value of Ka for a weak acid the {stronger/weaker} it is.
Stronger
The greater the pKa value for a weak acid, the {stronger/weaker} the acid is.
Weaker
A soluble salt of a strong acid and a strong base dissolves in water to produce a(n) {neutral/alkaline/acidic} solution.
Neutral
A soluble salt of a weak acid and a strong base dissolves in water to produce a(n) {neutral/alkaline/acidic} solution.
Alkaline
A soluble salt of a strong acid and a weak base dissolves in water to produce a(n) {neutral/alkaline/acidic} solution.
Acidic
Buffer solution
Solution in which the pH remains approximately constant when small amounts of acid, base or water are added.
An acid buffer consists of:
a solution of a weak acid and one of its salts made from a strong base
Explain how an acid buffer solution is able to maintain a constant pH despite the addition of an acid or base.
The salt of the weak acid provides the conjugate base, which can absorb excess hydrogen ions produced by the addition of a small amount of acid.
The weak acid molecules will dissociate and provide hydrogen ions to replace those removed by the addition of a small amount of base.
A basic buffer consists of:
a solution of a weak base and one of its salts
Explain how a basic buffer solution is able to maintain a constant pH despite the addition of an acid or base.
In a basic buffer solution the weak base removes excess hydrogen ions from the addition of an acid.
The conjugate acid provided by the salt supplies hydrogen ions when these are removed by hydroxide ions due to addition of a base.
Used to calculate an approximate pH of an acid buffer solution
Indicators
Weak acids, which when in aqueous solution, the colour of an acid indicator is distinctly different from that of its conjugate base.
Dissociation of indicators can be represented by:
HIn(aq) + H2O(l) <=> H3O+(aq) + In-(aq)
The acid indicator dissociation constant
The colour of the indicator is determined by the ratio of:
[HIn] to [In-]
The theoretical point at which colour change of an indicator occurs is when… Explain why.
The theoretical point at which colour change of an indicator occurs is when [H3O+] = KIn as KIn = [H3O+][In-]/ [HIn] and In- = HIn at endpoint. This is because when [H3O+] is large the equilibrium is shifted to the left and the colour will be of the weak acid, and when [H3O+] is small the opposite is true and the colour will be of the conjugate base.
pH = pKIn +/- 1
Used to estimate the range over which a colour change occurs
The colour change of an indicator is assumed to be distinguishable when:
[HIn] and [In−] differ by a factor of 10.
Explain why the correct choice of indicator is required for an acid-base titration
The equivalence point of an acid-base titration depends on the relative strengths of the acid and bases used. As the object of an acid-base titration is to determine the equivalence point as precisely as possible, it is important that the pH range of the indicator encompasses the pH of the equivalence point.
Explain why the pH of a buffer solution remains approximately constant when a small volume of water is added
The acid and salt will be diluted by the same amount so the ratio of acid to salt is unchanged