Water & Wastewater Design
1/56
Earn XP
Description and Tags
Wastewater
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
57 Terms
What are the common Water Quality parameters in wastewater treatments?
What are the three ways to describe the concentration of organic molecules in wastewater?
BOD, COD, & TOC
BOD
biochemical oxygen demand.
This measures the amount of oxygen needed by microorganisms to decompose organic matter in a specified time period, normally within a 5-day (BOD5) period at a constant temperature of 20°C.
Used to assess the strength of wastewater and the efficiency of biological treatment.
COD
Chemical Oxygen Demand.
This refers to the amount of oxygen required to chemically oxidize organic and inorganic substances in water, indicating the pollutant concentration.
Gives a faster estimate compared to BOD
This can be critical for industrial wastewater that may contain hard-to-degrade chemicals
TOC
Total Organic Carbon.
This measures the amount of carbon
direct and quick method to estimate total organic load.
What are the key atoms in bacterial cells, and what is the representative molecule?
The key atoms in bacterial cells are carbon, hydrogen, oxygen, nitrogen, phosphorus, and sulfur, with the representative molecule being DNA or RNA, which contains these atoms.
What are f_e and f_s, and how do they affect biological treatment?
f_e is the fraction of electrons that go to cell maintenance (max = 1). f_s fraction of electrons that go to synthesis (min=0).
f_e + f_s = 1
This reflects the balance between energy used for cell maintenance and new biomass production. The values influence:
Sludge yield (higher f_s means more sludge)
Oxygen demand (high f_e means more oxygen needed per unit substrate)
treatment efficiency (optimal balance improves degradation and system stability
What is the F:M ratio and how is it used?
The F:M ratio, or food-to-microorganism ratio, is a measurement used in wastewater treatment to assess the amount of organic substrate available for the microorganisms relative to their biomass. It influences the efficiency of biological treatment processes by determining optimal conditions for microbial growth.
What is Y and what affects its particular value?
Y, or yield coefficient, represents the amount of biomass produced per unit of substrate consumed in biological processes. Its value is influenced by factors such as temperature, pH, and nutrient availability.
4 types of biological treatments
Aerobic oxidation, Aerobic digestion, Anaerobic digestion, and Denitrification
Aerobic Oxidation
Electron donors: Organic molecules
electron acceptors: Oxygen
Bacteria Types: Heterotrophs
Reactors: Aeration Basin and Clarifiers
Conditions: pH- 6.9, DO>2 mg/L, and Needs and SRT of 3-5 days
Aerobic Nitrogen Removal
Electron Donors: Ammonia (NH3) and organic molecules
Electron Acceptors: Ozone (O3) and Nitrogen Gas (N2)
Bacteria Type: Autotroph
Conditions: pH 7.5-8, DO > 4 mg/L, SRT of 20 days
Reactors: Aeration Basin, and Clarifiers
Denitrification
Electron Donors: Organic matter
Electron Acceptors: NO3-
Bacteria Types: heterotrophs
Conditions: anoxic environment, pH 6.5-8.5, and SRT of 10-30 days. Needs nitrate and no O2
Reactors: Anoxic Basins.
Anaerobic Digestion
A biological process that breaks down organic matter in the absence of oxygen, resulting in biogas production. It is typically performed by anaerobic bacteria and involves conditions such as a pH of 6.8-8.0 and SRT of 15-30 days.
Why is SRT important?
Solids Retention Time, is crucial for biological treatment as it influences microbial population dynamics, nutrient removal efficiency, and overall system stability. Adjusting SRT can enhance or inhibit the growth of specific microbial communities, impacting treatment performance.
What are some operational issues that can occur at the secondary clarifier related to sludge settling?
Operational issues in secondary clarifiers can include poor sludge settling, bulking, scum formation, and varying sludge blanket levels that affect overall treatment efficiency.
What are two main categories of solids handling?
Landfill and Land application
Landfill
A method of disposing solid waste by burying it in the ground, often in a designated area, to minimize environmental impact. Sludge from the primary clairfier.
Land application
A method of recycling organic waste, such as biosolids, by applying it to land for beneficial use, typically enhancing soil quality and fertility. The sludge sent here is primarily from the secondary clarifier.
Class A Solids
PRFP (process further reduce pathogens) composting that needs a temperature of >55°C
heat drying- moisture content >10%
Thermophilic aerobic digestion >10days at >60C
Beta Ray Irradiation, Gamma Ray Irradiation, and Pasteurization> 70 °C for 30 minutes.
Can be used for direct Agriculture and sold to the public.
Class B
PSRP (Process Significantly Reduce Pathogens)
Composting: >40C for >5days
Air dry: > 3 months
Aerobic digestion: >40 days SRT at 20°C or 60 days at 15C.
Anaerobic digestion: 15 days at 35 °C or 60 days at 20 °C
Lime Stabilization: kill pathogens once pH > 12 after 2 hours.
Can only be applied for indirect agriculture applications (hay fields).
types of stabilization
Results are in a non-degradable or dangerous form. This can include digestion, chemical stabilization, and thermal stabilization.
Digestion Stabilization
This is designed to reduce the amount of solids in a wastewater treatment process by breaking down the organic matter into simpler substances through microbial activity, leading to reduced volume and odor.
Chemical Stabilization
A process that involves using chemicals to reduce the pathogens in sludge, making it safer for storage and application. It often involves the addition of lime or other agents to alter the pH and stabilize the organic matter.
Thermal Stabilization
A treatment process that uses heat to reduce pathogens and organic matter in sludge, resulting in a more stable and less odorous final product.
types of wastewater reuse
Defacto, Direct, & indirect
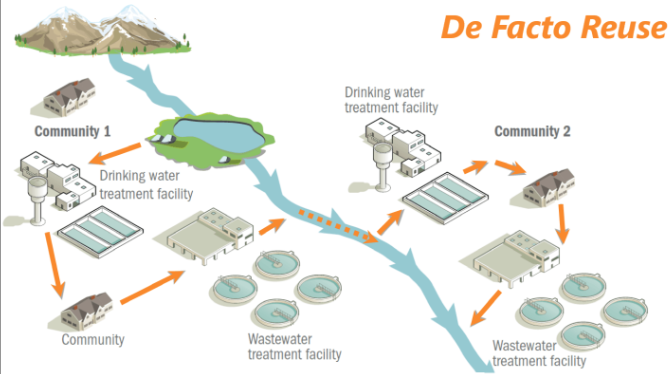
Defacto
wastewater plant → natural body of water → drinking water treatment plant → consumers and starts all over again.
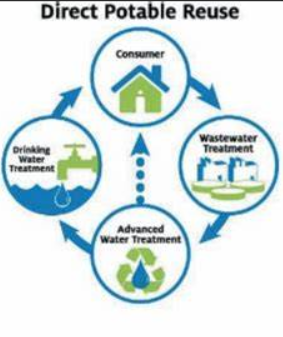
Direct reuse of water
consumer → wastewater plant → advanced treatment → drinking water treatment, and starts all over again.
Advanced treatment will also go straight to the consumers.
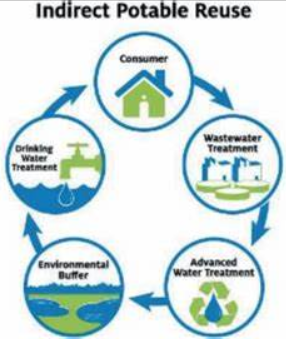
Indirect Ruse of Water
consumer → wastewater plant → advance water treatment → environment buffer → drinking water plant and starts all over again.
What are the 2 main water constituents?
Pathogens and Chemical Sources.
Pathogens
ex. bacteria, viruses, and protozoa (Giardia and cryptosporidium)
Chemical Sources
Can come from: industrial sites, domestic, human-based, or DBPs.
Biological Active Filtration
media filtration allows/ encourages bacterial growth.
also allows for increased treatment, but this is a slow process.
Granular Activated Carbon
practically a Brita filter.
this is good at removing organics and certain metals, but not very good at small ions.
Ion Exchange
this is similar to granular activated carbon, but this process has beads with “pre-loaded” ions that can be exchanged.
Peracetic Acid (PAA)
disinfectant that is commonly used in the food industry. (this is vinegar btw)
creates no residual or DBP’s
breaks down into acetic acid, water, and oxygen.
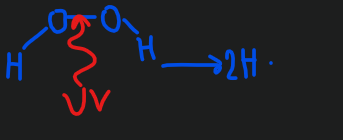
Advance Oxidation Processes
this makes free radicals (molecules with extra electrons)
ex. uv + hydrogen peroxide
Aquifer strategies
Enhanced aquifer storage (EAR)
both inject and pump out from the same aquifer.
injection wells
shallow wells
infiltration wells
Biological Phosphorous
not typically required
cells will eat some but not enough to meet permit. They can be removed chemically, but this is expensive.
PAO
Phosphorus-accumulating organisms. These store high quantities of P as poly-P.
already present in the aeration basin, but may not accumulate phosphorous.
Volatile Fatty Acid
These go dormant in an anaerobic basin
will release stored phosphorus into the water.
Non - PAO’s
Could have used the VFA as substrate, but the PAOs got to it first. And over time PAOs will dominate the activated sludge population.
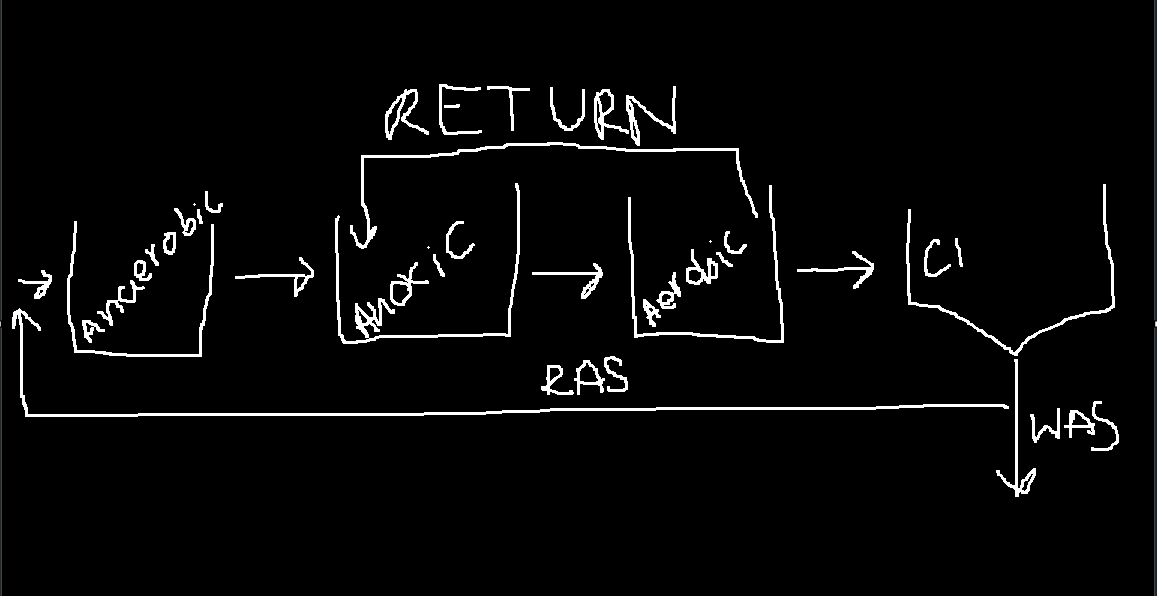
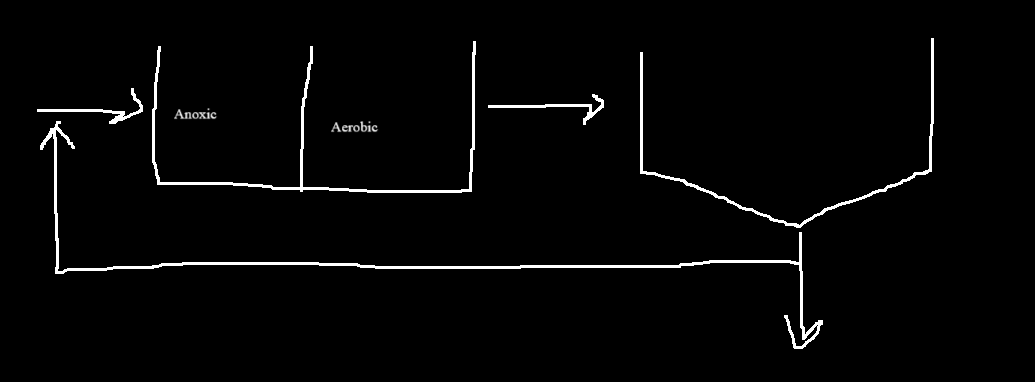
A²/O
With return before exit, little to no nitrate makes its way to anaerobic.
This allows for Nitrification, Denitrification, and P removal
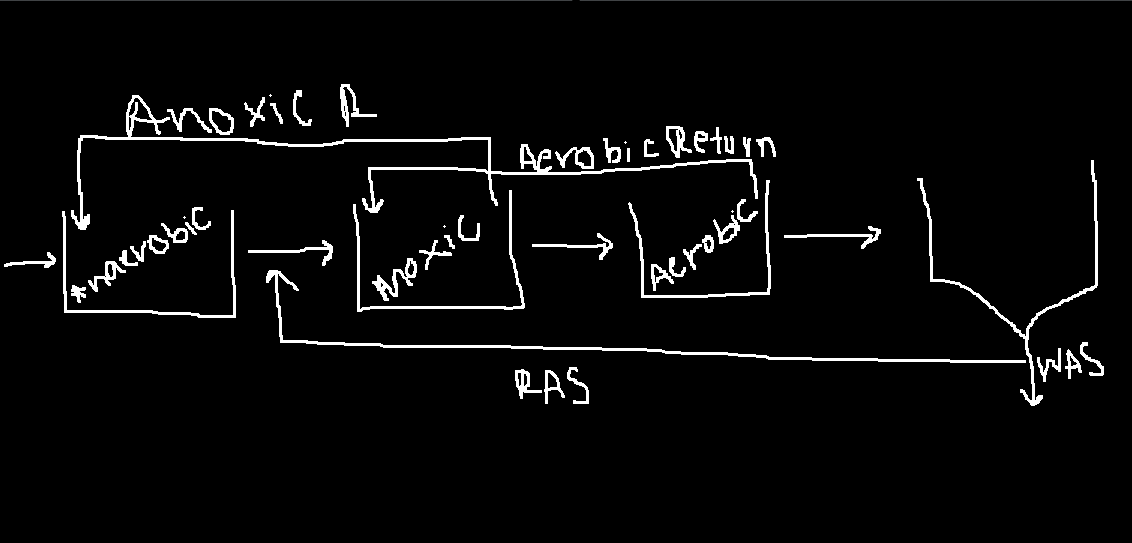
UCT
useful if you are concerned about nitrogen popping up in the anerobic reactor.
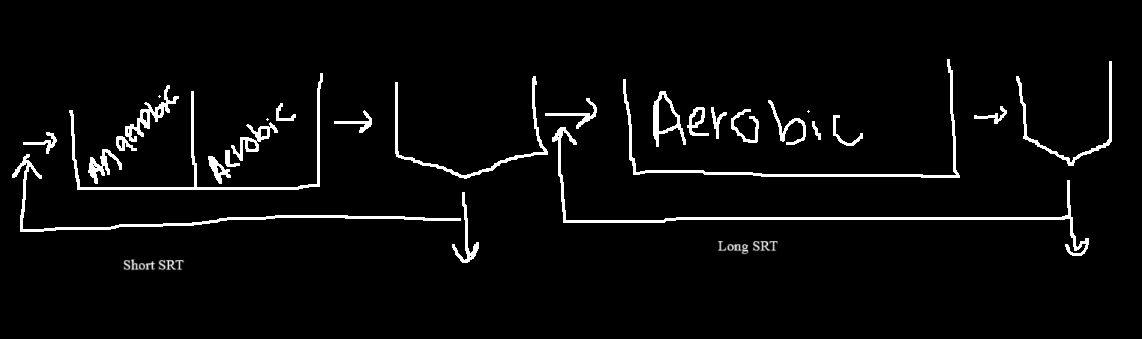
How to Stop Nitrification
Since nitrification grows slowly, we can decrease the SRT, this and also having a high WAS rate will prevent a nitrifier from forming.
Nitrification/ Denitrification
anaerobic chambers can turn anoxic
PAOs don’t uptake VFA or release P, but you don’t get any P removal.
can remove organics, NO3, and NH3
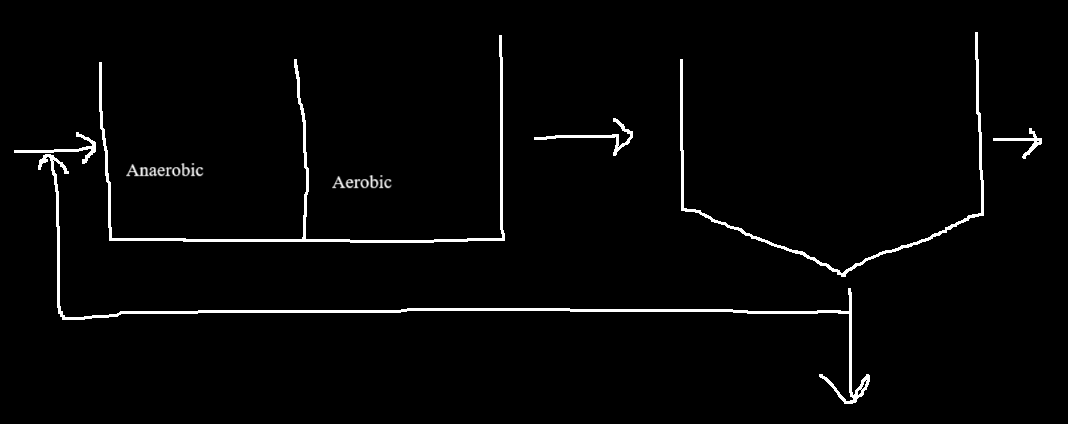
AO system
In the anaerobic chamber the non-PAOs go dormant.
this allows for the PAOs to use simple organics (VFAs) as electron acceptors.
can remove organics, NH3, and phosphorous.
Filamentous Bacteria
A type of “Bulking Sludge” that will creates long chains that will not settle out. These grow at low DO (dissolved oxygen) and low F/M (food/microbe)
Avoid: low oxygen and low food levels
Viscous Bulking
A type of “Bulking Sludge” that will release sticky compounds that allow for surface attachment.
Avoid: high food ratio
Nocardioform Foam
A type of bacteria that will release hydrophobic compounds.
foam build up that is trapped. - Bottom of the clarifier is anoxic, bacteria produce N2 bubbles, and “rising sludge” is produced.
Primary Sludge
“Large” organic matter
inorganic matter
both of these are from influent.
Secondary Sludge
Biomass
Small inorganic
handled separately
better for land application
Hydrolysis
Happens in anaerobic digestion, where it takes larger molecules and breaks it down into smaller pieces.
this requires extra cellular enzymes
This process is energy intensive and slow
aided if some thickening has occurred.
Acidogenesis
Creation of VFAs (volatile fatty acids) and also acetic acids.
energy producing, but still slow, this will produce new cells.
Methanogenesis
different group of cells (bacteria)
further converts acetic acid into CH4
It can be captured and burned to run a generator.
ThOD
Theoretical Oxygen Demand.
Calculated oxygen demand based on a formula.