Sigma and pi bonds + alkene reactivity
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Compare the bond enthalpy of sigma and pi bonds
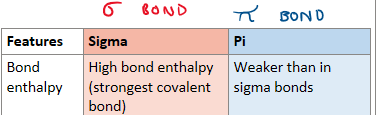
Why do sigma bonds have a higher bond enthalpy?
Due to the strong electrostatic attraction between the nuclei and shared pair of electrons due to the high electron density between nuclei
Why do pi bonds have a lower enthalpy?
Electron density is spread out above and below the nuclei HENCE electrostatic attraction is weaker
Which orbitals overlap in a sigma bond?
2 s-orbitals overlap (they align horizontally and give a single covalent bond)
Which orbitals overlap in a pi bond?
2 p-orbitals overlap in parallel
What type of bond is between C-C and C-H?
Pi bond
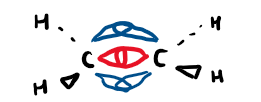
Draw the sigma and pi bonds in C=C
red = sigma
blue = pi
Why are alkenes more reactive than alkanes?
Alkenes are more reactive than alkanes due to their double bond being a high electron density area.
How are alkenes open to attack by electrophiles?
Pi bonds stick out a little and the whole double bond has a high electron density -> open to attack by electrophiles