Unit 2 Lectures 2, 3 & 4 - The Lipid Bilayer
1/76
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
77 Terms
Features of biological membranes
1. Bilayer of phospholipids
2. Organized and fluid
3. Different permeability for different types of molecules
4. Asymmetric
Role of lipids in the membrane
- impermeable barrier to water-soluble materials
- electrical resistance
- flexibility
- stability
Types of Lipids
1. Phospholipids
2. Sterols
3. Fatty Acids
4. Triacylglyerols
Why do phospholipids form a bilayer?
Hydrophilic heads attracted by water so are on the outside
Hydrophobic tails repel water so point inwards
What form do fatty acids take in aqueous environments?
micelle
What forms do phospholipids take in aqueous environments?
bilayer and liposome
What form do sterols take in aqueous environments?
monolayer on their own with the hydroxyl group interacting with water
they can be inserted into the phospholipid bilayer
What form do triacylglycerides take in aqueous environments?
oil droplets
Requirements for bilayer formation
1. Amphipathicity
2. Correct shape (head + 2 tails makes cylinder shape)
Thermodynamics of the hydrophobic effect (minimum energy conformation)
the minimum energy conformation is achieved by minimizing the exposure of hydrophobic groups to water
Thermodynamics of the hydrophobic effect (free energy)
the free energy of the system is minimized if the hydrophobic regions cluster together to limit contact with water
Thermodynamics of the hydrophobic effect (motional freedom)
water achieves the greatest possible motional freedom if its contact is limited with hydrophobic groups
Why is there no water within the hydrophobic core of the bilayer
water cannot hydrogen bond with the fatty acid tail, causing it to be shielded from the fatty acid core by the polar heads
Biological membrane makeup
phospholipids, glycolipids, sterols
Lateral diffusion within the fluid mosaic model
phospholipids can diffuse laterally because, in doing so, they remain at the same energy level and are under the same conditions
Transverse diffusion within the fluid mosaic model
It requires a lot of energy for polar head groups to move through the hydrophobic section of the lipid bilayer; flippases make this possible
FRAP
Fluorescent Recovery After Photobleaching
What does FRAP measure?
rate of diffusion of membrane proteins (lateral recovery)
How does FRAP work?
1. fluorescent marker is bound to the phospholipids that make up a cell membrane.
2. laser is used to bleach a small patch of membrane (stop the patch from fluorescing)
3. patch will again show fluorescence due to lateral diffusion of phospholipids throughout the membrane OR no fluorescence will be shown
What does it mean when a patch recovers quickly from bleaching?
the membrane is more fluid/mobile; it's proteins are not anchored
What does it mean when a patch recovers slowly from bleaching?
the membrane is less fluid/mobile; it's proteins are anchored
Effect of temperature on lipids
at cold temperatures, membrane bilayers freeze and assume a crystalline state with low fluidity and high fragility
[Plants and fungi/Animals] use tactics to maintain fluidity more often?
Plants and fungi; they are less capable of moving to a warmer space
Tactics to increase fluidity
- increase number of unsaturated lipids
- decrease tail length
- increase amount of sterols
How does increasing the number of unsaturated lipids maintain fluidity?
a higher number of saturated lipids means that the bilayer is more tightly packed with more van der Waals interactions and is less fluid; the fluidity can be increased by increasing the amount of unsaturated fatty acids since there are more kinks in the tails
How does decreasing tail length maintain fluidity?
shorter tails are more fluid since they have less surface area to form van der Waals interactions
How does changing the amount of sterols maintain fluidity?
- at low temperatures, sterols decrease van der Waals and increase fluidity
- at high temperatures, sterols introduce more surface area and more van der Waals interactions, leaving the membrane less fluid
Lipid Rafts
lipid-rich microdomains that are more ordered than the supporting membrane
Methods of membrane adhesion to other molecules
- adhesion to proteins inside the cell
- adhesion to extracellular matrix
- adhesion to neighbouring cells
- adhesions to proteins inside the cell (in the cell cortex)
The plasma membrane is reinforced inside the cell by
association of membrane proteins with the cell cortex
Adhesion to extracellular matrix
integrin proteins can connect to the extracellular matrix
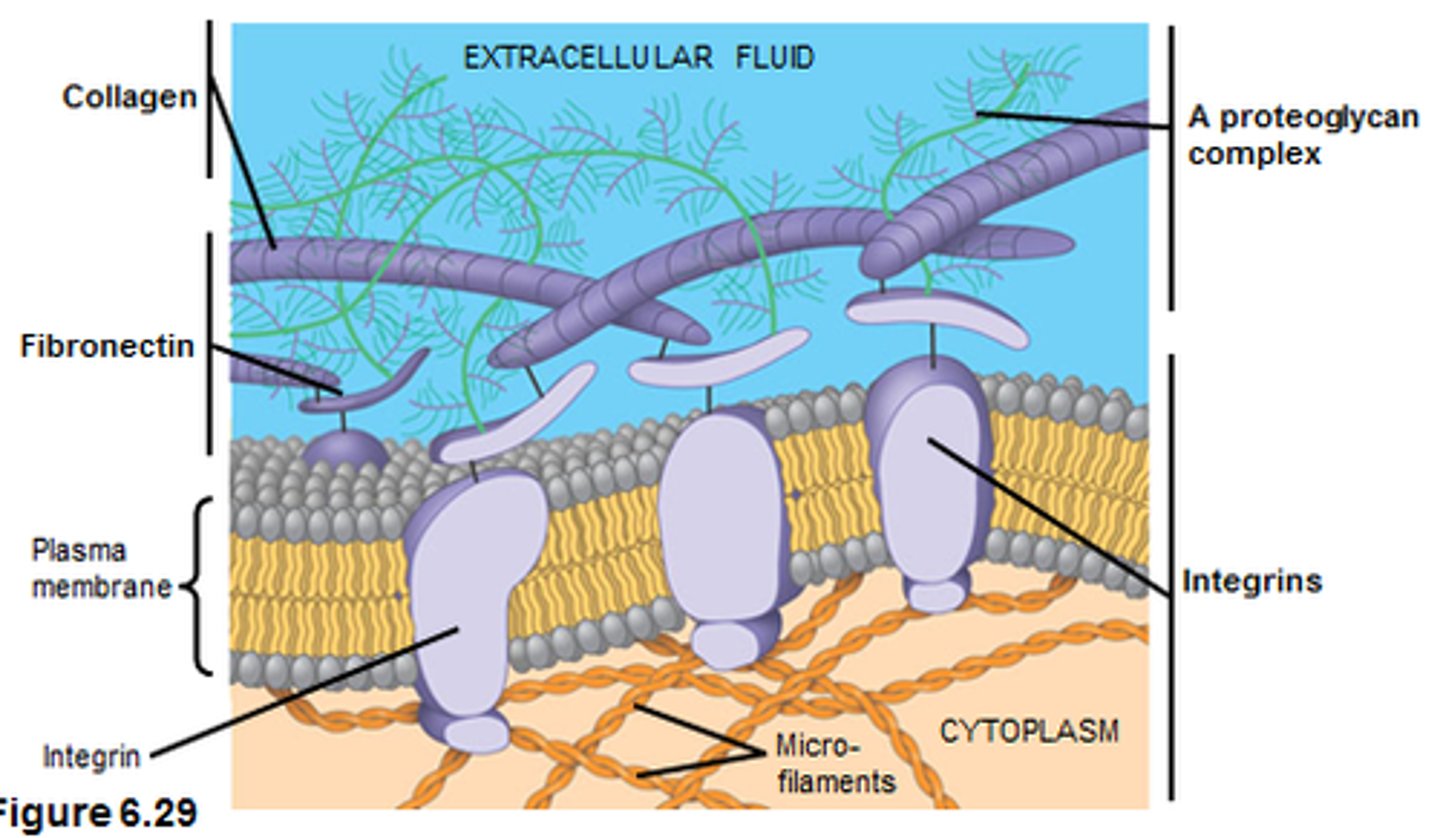
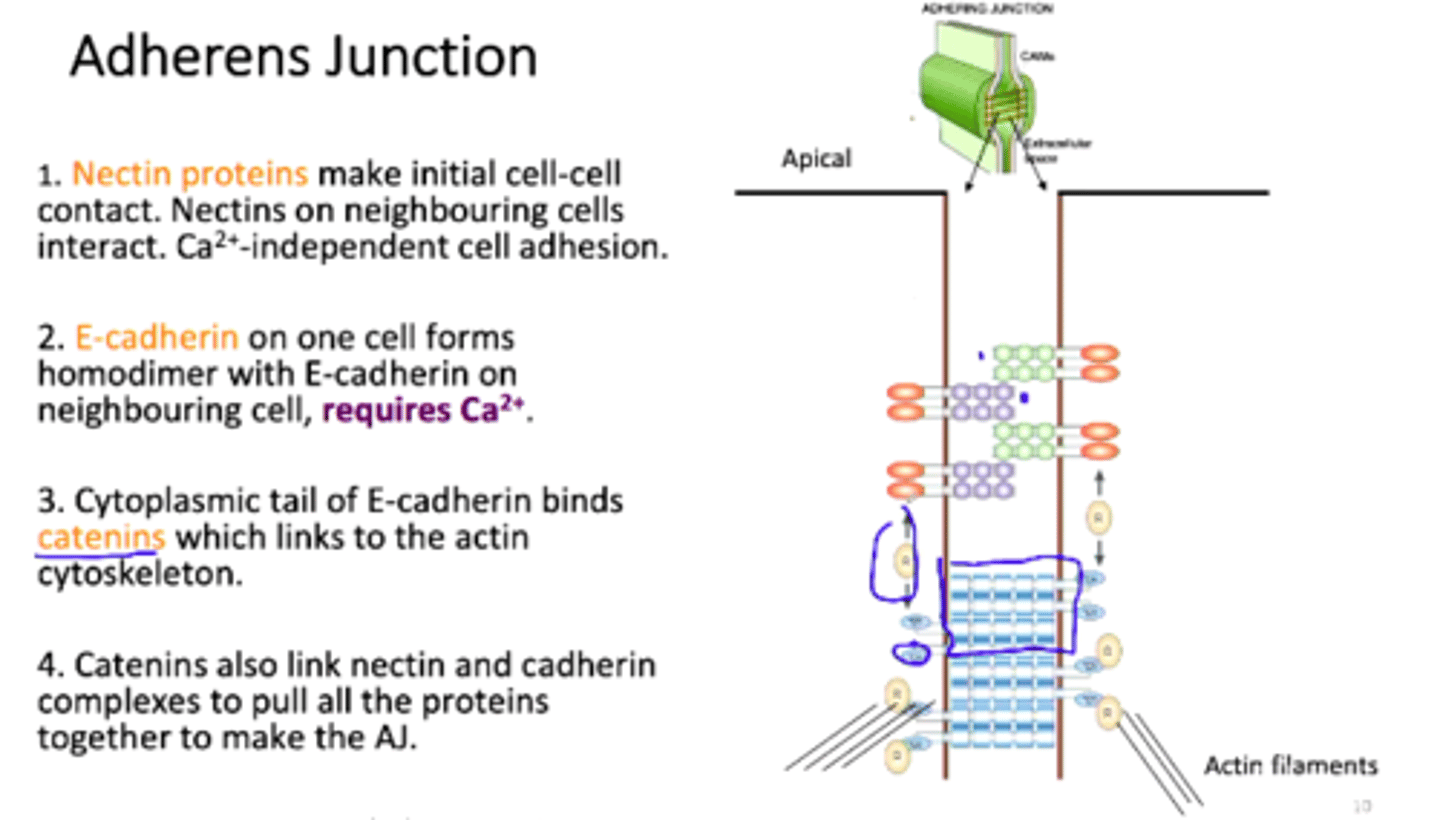
Adhesion to neighbouring cells
cell to cell adhesion molecules (cadherins) linking the plasma membranes of neuronal cells
Tight junctions
- adhesions between neighbouring epithelial cells so nothing leaks between the cells
- segregates apical and basolateral sides of cells into distinct membrane domains
- especially important for cells in the intestinal epithelium (leaky gut)
Faulty tight junctions
permits undigested food particles, microorganisms, and toxins to pass through the epithelium into capillaries (leaky gut)
Anchored proteins with FRAP
after photobleaching, the area of a protein with anchored proteins will remain bleached. this is because the protein is anchored and cannot undergo lateral diffusion.
Unanchored proteins with FRAP
after photobleaching, the area of a protein with unanchored proteins will regain brightness. this indicates that the membrane is fluid and proteins are able to disperse via lateral diffusion.
Permeability of the lipid bilayer for gases and hydrophobic molecules
diffuse freely
Permeability of the lipid bilayer for small uncharged polar molecules
diffuse fairly well
Permeability of the lipid bilayer for large, uncharged, polar molecules
diffusion is negligible
Permeability of the lipid bilayer for ions
diffusion is not possible
How do proteins change membrane permeability?
proteins allow the cell to be selectively permeable; each organelle has its own subset of unique channels and carriers
N terminus
the end of a polypeptide or protein that has a free amino group
C terminus
the end of a polypeptide or protein that has a free carboxyl group
Amino acid residue
an amino acid that is part of a peptide, polypeptide, or protein chain
Bonding/interactions involved in primary structure
covalent (peptide) bonds between amino acids
Bonding/interactions involved in secondary structure
backbone interactions via hydrogen bonding
Alpha helix
- repetitive hydrogen bonds all the way along and parallel to the backbone of the helix
- r groups project outward
Beta pleated sheets
- can be parallel or antiparallel
- r groups project upward or downward away from the peptide backbone
Sides of the beta pleated sheet are [identical/distinct]
distinct
Secondary structure elements fold into ___________ within a tertiary structure
domains
Different domains are associated with different functions, including
- transmembrane domain
- DNA binding domain
- catalytic domain
Bonding/interactions involved in tertiary and quaternary structure
- hydrogen bonds
- ionic bonds
- van der Waals interactions
- disulfide bonds
Quaternary structure stabilization
- stabilized by all factors that stabilize the tertiary structure
- the 3D configuration of different polypeptides that make up a molecular complex consisting of several subunits
- stabilized by a combination of hydrophilic or hydrophobic interactions (or both) between polypeptides
Amino acid side chains on the side of a final, folded protein complex
hydrophilic
Protein structure formation thermodynamics
- increase the stability of the system by having non-covalent and covalent interactions
- allow for molecular self-assembly
Primary structure
linear amino acid sequence of peptide-bonded amino acids; determines the protein's 3D structure
Secondary structure
local 3-D structure stabilized by backbone H-bonding of the peptide; examples include alpha-helices and beta-sheets
Tertiary Structure
overall 3-D structure ('fold') of entirepolypeptide; stabilized by side-chain interactions (non-covalent anddisulfide bonds) as well as interactions between side chains andbackbone atoms
Quaternary structure
3-D arrangement of polypeptides in aprotein composed of multiple subunits; similar stabilization astertiary
Integral membrane proteins
Proteins directly attached to the membrane ;amphipathic; can be monomeric or multimeric
Peripheral membrane proteins
Bound to membrane surfaces through non-covalent association with other membrane proteins
Asymmetry with integral membrane proteins
the orientation of transmembrane proteins matter; the leaflet of attachment matters
Asymmetry with peripheral membrane proteins
different proteins attach to different sides
Hydropathy plots
plots measuring the hydrophobicity of proteins; peaks above the threshold indicate potential transmembrane alpha helices; used to predict the number and orientation of alpha helical transmembrane segments
Characteristics of a membrane spanning domain?
non-polar section for the interior of the membrane to interact with phospholipid tails; polar section for the exterior of the membrane to interact with the polar heads
Peripheral protein attachment to membrane
attached indirectly through non-covalent interactions
Isolating peripheral proteins
use high salt to weaken protein-protein interactions by disrupting electrostatic bonds
Integral protein attachment to membrane
van der Waals interactions
Isolating integral membrane proteins
use detergent (SDS, Triton X-100) to solubilize the proteins
Protein electrophoresis gel
polyacrylamide
DNA electrophoresis gel
agarose
Protein electrophoresis
- different charges and shapes because of amino acid sequence
- a strong ionic detergent helps equalize the charge-to-mass ratio and also denatures proteins so that they separate according to size
DNA electrophoresis
- equal charge-to-mass ratio throughout the length of each molecule
- separates according to size
Membrane asymmetry
different lipids in the extracellular side and the cytosolic side
Flippases
specific membrane proteins that maintain the bidirectional transport of lipids between the layers of the phospholipid bilayer in cells
Floppases
move amphiphilic lipids from inner leaflet to outer leaflet
ATP dependent
Scramblases
remove randomly selected phospholipids from one half of the lipid bilayer and insert them in the other