RX 523: Final Exam
1/1974
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
1975 Terms
The success of drug therapy is highly dependent upon what?
the drug selection, the drug product (dosage form) administered, and the design of the drug dosing regimen
The drug dosing regimen is designed to achieve what?
a target drug concentration at the site of action to elicit an optimal therapeutic response while minimizing adverse effects
To achieve the desired drug concentration, numerous factors must be considered including what?
a thorough patient evaluation and an assessment of the pharmacokinetic and pharmacodynamic characteristics of the drug
How is a pharmacist's role vital to drug therapy regimens?
Pharmacists perform vital roles in the collection and assessment of patient data and the evaluation of drug information utilized in the design of individual drug therapy regimens
Biopharmaceutics
The interrelationship of the physiochemical properties of a drug, the dosage form in which the drug is given, and the route of administration on the rate and extent of systemic absorption
Pharmacokinetics
Study of the time course of drug processes that determine the concentration of drugs in body fluid and tissues over time (absorption, distribution, biotransformation (aka metabolism), and excretion
Clinical Pharmacokinetics
The application of pharmacokinetic principles to the safe and effective therapeutic management of drugs in an individual patient
Pharmacodynamics
The study of the detailed mechanism of action by which drugs produce their pharmacologic effect. Pharmacodynamics refers to the relationship between the drug concentration at the site of action and the resulting effect
Therapeutic Drug Monitoring
Measurement of drug concentrations and assessment and application of the resulting concentrations to design safe and effective drug regimens
The application of the pharmacokinetic principles requires what?
an understanding of the absorption, distribution, metabolism, and excretion characteristics of specific drugs utilized in specific disease states and various patient populations.
How is the Pharmacists' knowledge of the fundamental pharmacokinetic principles vital?
It is vital in the design of dosage regimens of specific drug products for individual patients to enhance pharmacotherapeutic efficacy and minimize toxicity of an individual patient's drug therapy
What must be identified and incorporated into the drug regimen design?
The existence and influence of any factors that may introduce variability in patient pharmacokinetic process(es), parameters, plasma drug concentrations, and ultimately the patient response to drug therapy.
Examples of Pharmacokinetic Variability in patients
- Age
- Sex
- Genetics
- Body Composition
- Weight
- Nutritional Status
- Pathophysiology
- Medical & Surgical history
- Concomitant medications
- Environment
- Social history
Pharmacokinetic Variability in patients
The patient's pharmacokinetic process(es), parameters, plasma drug concentrations, and ultimate response to drug therapy may vary considerably among individuals receiving the same drug therapy regimen Interpatient and intrapatient variability may exist regarding pharmacokinetic and pharmacodynamic principles
Population Versus Patient-Specific Pharmacokinetics: Population Pharmacokinetics
- The study of the sources and correlates of variability in drug concentrations among individuals who are the target population receiving clinically relevant doses of a drug of interest
- Population pharmacokinetics seeks to identify the measurable pathophysiologic factors that cause changes in the dose-concentration relationship and the extent of these changes so that, if such changes are associated with clinically significant shifts in the therapeutic range, dosage can be appropriately modified
- Pharmacokinetic studies typically include healthy volunteers or a specific patient population
- Study results commonly include mean plasma drug concentration versus time curve profile data (mean concentrations, volume of distribution, half-life, clearance values, area under the curve)
- Population pharmacokinetic parameter data is often utilized to create an initial/empiric drug therapy regimen
Population Versus Patient-Specific Pharmacokinetics: Patient-Specific Pharmacokinetics
- Patient-specific pharmacokinetics represents the utilization of patient-specific data to design and/or adjust patient drug therapy regimens
- Commonly, this may include the use of plasma drug concentrations obtained from a specific patient
- Pharmacokinetic calculations may be performed to characterize pharmacokinetic parameters specific to your patient
- Patient-specific pharmacokinetic parameter data is often utilized to perform drug therapy regimen adjustments
- Always use patient-specific pharmacokinetic data when it is available to integrate plasma drug concentrations into the patient's therapeutic goals, clinical response, and desired outcome for therapy
What is a fundamental responsibility of all pharmacists providing pharmaceutical care?
Clinical pharmacokinetic monitoring
The pharmaceutical care process requires pharmacists to do what?
perform functions in the clinical capacity and includes therapeutic drug monitoring and the provision of clinical pharmacokinetic assessments
Clinical pharmacokinetic monitoring is essential for what?
to achieving positive outcomes for patients across the continuum of care and in all practice settings of health systems
How is the ever-increasing roles of pharmacists associated with clinical pharmacokinetics
Pharmacist-based medication management (e.g., vancomycin, aminoglycosides, anticoagulation, antimicrobial selection, renal dysfunction dose adjustment, nutrition, etc.) Authorization to order drug concentrations, laboratory parameters, pharmacogenetic testing, medications
Pharmacists' Clinical Functions in Clinical Pharmacokinetics
- Design dosage regimens
- Recommending and/or scheduling measurements
- Monitor, evaluate & adjust regimens
- Communicate
- Educate
- Promote collaboration
TDM
Therapeutic Drug Monitoring
Therapeutic Drug Monitoring (TDM)
Measurement of drug concentrations and assessment and application of the resulting concentrations to design safe and effective drug regimens
What is the goal of TDM?
to obtain appropriate drug concentrations to yield confident estimates of pharmacokinetic parameters
Support for the Utilization of TDM
- Good correlation exists between the pharmacologic response and the plasma concentration
- Wide interpatient variability in plasma drug concentration occur following a given dose
- The drug has a narrow therapeutic window/range
- The desired effect of the drug cannot be observed by other readily available methods/means (i.e. surrogate parameters)
Clinical Pharmacokinetics & The Pharmacists' Patient Care Process (PPCP)
- Collect data from the patient and medical record
- Assess patient data, including drug concentrations, according to patient therapeutic goals and desired outcome
- Part of your assessment and plan may include, when appropriate, performing pharmacokinetic calculations to develop your plan of care
- Create a complete drug therapy plan
- Implement the drug therapy plan
- Create a monitoring plan and follow-up to determine achievement of patient therapeutic goals and desired outcome
- Communicate assessment plan to appropriate healthcare professionals, patient, and/or caregiver(s)
The Pharmacokinetic Consult for Pharmacists
- Pharmacist-based medication management
- Pharmacist' role in TDM for medications monitored via drug concentration(s)
- Pharmacy to monitor/evaluate/adjust drug therapy based upon decreased renal function, IV to PO dosing protocol, nutrition requirements, pharmacogenomic testing etc.
- Documentation of communications, recommendations, and/or interventions in the patient medical record
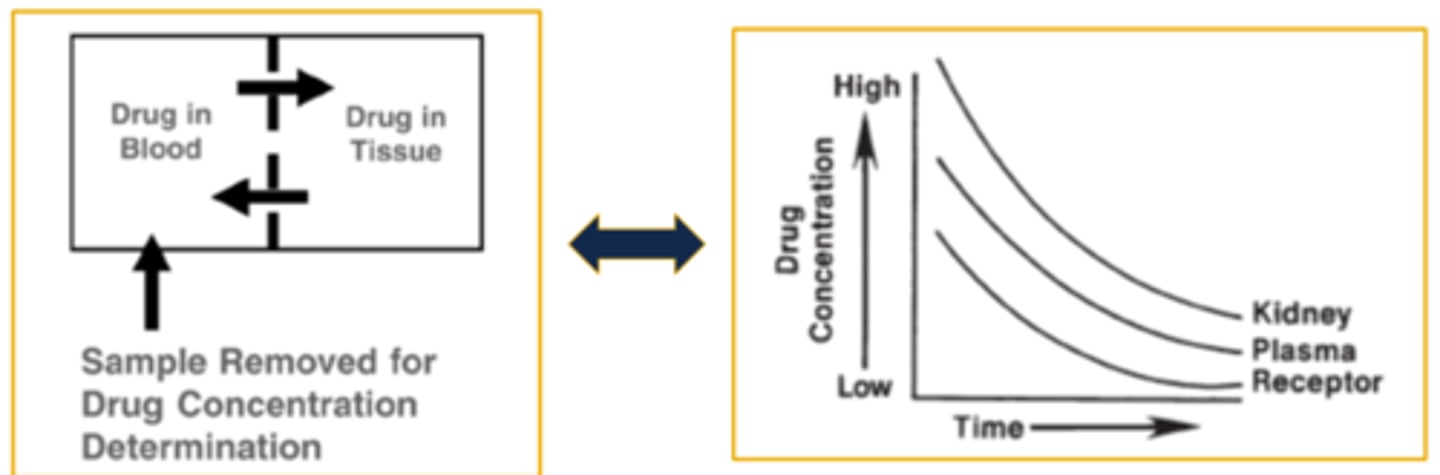
Plasma Drug Concentration Versus Time Curve
- The curve is generated by collecting samples from the patient and measuring the concentration of drug at various time intervals following dose administration
- Measuring drug concentrations (a.k.a. levels) from the patient's blood, serum, or plasma allows us to quantify, study, assess, and calculate pharmacokinetic parameters related to absorption, distribution, and elimination
- The concentration (y-axis) is plotted against the time (x-axis) at which the sample was collected from the patient
- When two concentrations are collected appropriately a best fit line/curve develops and predictions may be made for concentrations at any point in time along the curve
- The relationship between the concentration and time enable the extraction and/or calculation of parameter data from the curve
The Utility of Measuring Drug Concentrations
1. A drug's pharmacologic effect is related to its concentration at the site of action (i.e., receptor site)
2. Direct measurement of drug concentrations at the site of action are neither safe nor practical in most cases
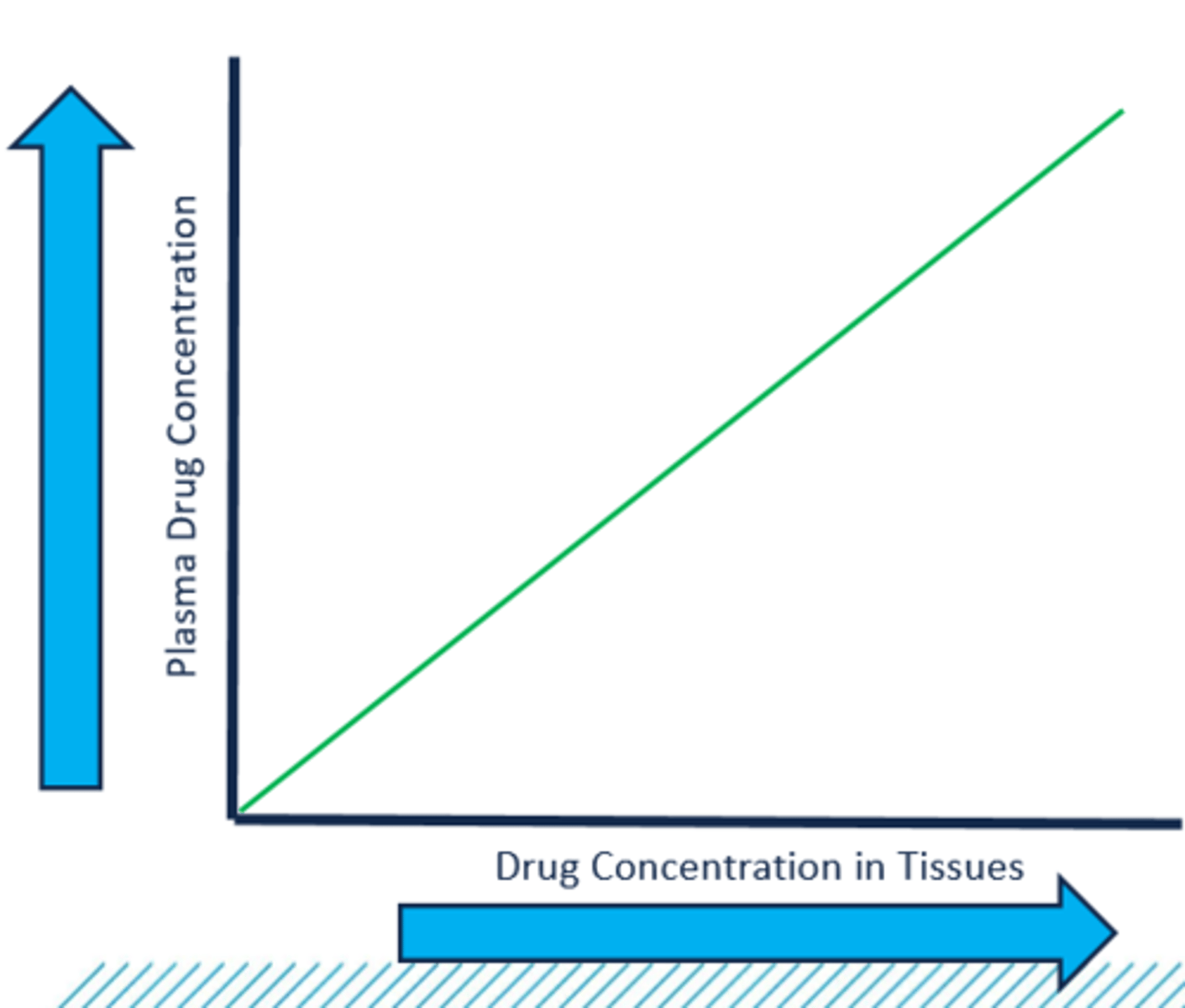
3. The concept of kinetic homogeneity describes the predictable relationship between plasma drug concentration and the concentration at the site of action
4. In the practice of clinical pharmacokinetics, we assume that the plasma drug concentrations directly relate to concentrations in the tissues where the disease process is affected by the drug
kinetic homogeneity
describes the predictable relationship between plasma drug concentration and the concentration at the site of action
Plasma Drug Concentrations & Kinetic Homogeneity graph (image)
Plasma Drug Concentration Time Curve Onset
The minute the conc hits the MEC
Plasma Drug Concentration Time Curve Offset
The minute the drug conc reaches the mec
MEC
minimal effective concentration
MTC
minimal toxic concentration
Preparing to Extract and/or Calculate Data from the Curve
- Mathematically speaking, it is easier to predict plasma drug concentrations at various times during the drug dose exposure if the concentrations are plotted on a straight line rather than a curved line
--- Converting the concentration versus time curve is accomplished by taking the natural log (ln) of the plasma concentration (y-axis)
--- Calculating pharmacokinetic parameters is conducted with the use of semi-logarithmic graphing practices
--- You will notice that we obtain a straight line using semi-logarithmic graphing paper only when the drug follows 1storder elimination processes
- Utilizing semi-logarithmic graph paper, we "linearize" the data points/plotted drug concentration collected from a patient, and we can perform calculations to predict plasma drug concentrations at any point along the curve
Pharmacotherapeutics
Medical science concerned with the use of drugs in the treatment of disease
Primary goal of Pharmacotherapeutics
to achieve an optimal beneficial effect with minimal adverse effectsIncorporating the principles of pharmacokinetics and pharmacodynamics will facilitate the achievement of this goal
Primary goal of Clinical Pharmacokinetics
to enhance the efficacy and decrease the toxicity of an individual patient's drug therapy regimen. Additional goals include assessing adherence and indirectly assessing organ function
The Foundational Pharmacokinetic Principles
- Absorption
- Distribution
- Elimination
Absorption
- Movement of drug molecules from the site of administration into the bloodstream (circulation) - Requires drugs to cross one or more layers of cells and cell membranes
Distribution
- Process of drug leaving the plasma/bloodstream
- Movement of drug into additional body fluids, tissues, and/or organs
Elimination
- Biotransformation to one or more metabolites
- Excretion of the parent drug and/or metabolites
Pharmacodynamic Concepts
The study of the detailed mechanism of action by which drugs produce their pharmacologic effect. Pharmacodynamics refers to the relationship between the drug concentration at the site of action and the resulting effect
Dose-response curve =
the relationship between drug dose and the magnitude of the pharmacologic effect
The Relationship Between Pharmacokinetics (PK) & Pharmacodynamics (PD) (image)
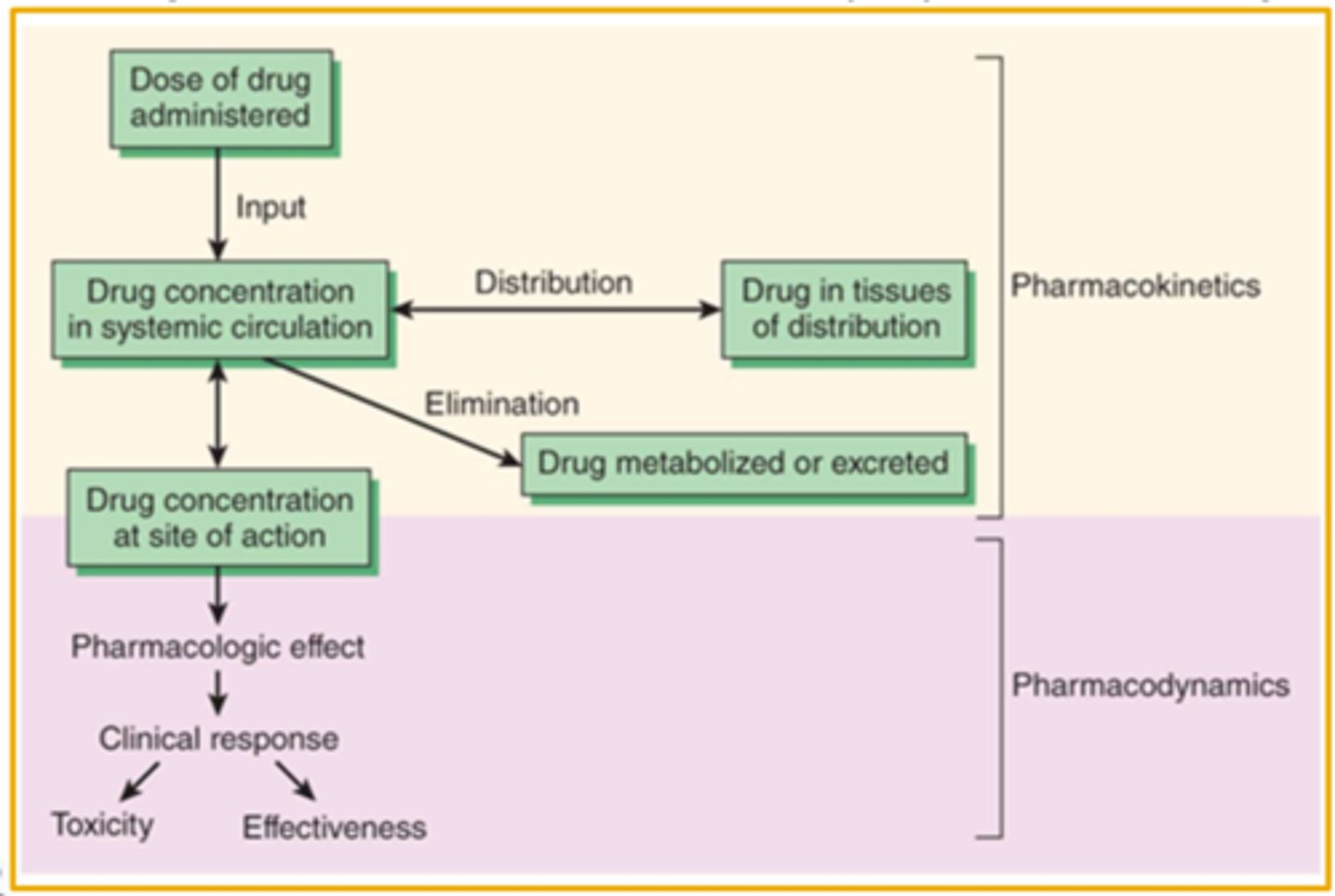
The Concentration is the Connection (image)
Pharmacokinetic Process Order
1. The pharmacokinetic processes alter drug concentration throughout the body over time
2. Absorption, distribution, metabolism, and excretion (ADME) processes are associated with a rate
3. The rate of a process is related to the specific order by which the process proceeds (First-Order and Zero-Order)
First Versus Zero-Order Pharmacokinetic Processes - Elimination: First-Order
- The amount of drug eliminated over time is directly proportional to the amount of drug in the body
- The amount eliminated with increase if the concentration increases and decrease if the concentration decreases
- The fraction/percentage of drug eliminated over a given time will remain constant
- The actual amount eliminated will differ, but the fraction is the same
First Versus Zero-Order Pharmacokinetic Processes - Elimination: Zero-Order
- The amount of drug eliminated over a given time does not change with the amount of drug/concentration in the body
- The amount of drug eliminated remains the same
- The fraction of drug eliminated will differ, but the amount is the same
The Need to Employ Pharmacokinetic Models
- Pharmacokinetic processes of absorption, distribution, and elimination are complex and often occurring simultaneously
- The inherent complexity of the changes to drug concentration throughout the kinetic processes necessitates the use of mathematical models and statistics
- A model is a hypothesis using mathematical terms to describe quantitative relationships in a concise manner
--- Models employ assumptions to reduce complexity (i.e., simplification) concerning the movement of drug throughout the body
--- Models lead to better understand the relationship between drug concentrations and response
--- Models help explain observations and make predictions
Types of Pharmacokinetic Models
- Compartment Model
- Physiologic Based Pharmacokinetic Model
- Population Pharmacokinetic Models
- Non-Compartmental (Model-Independent)
Functions of Pharmacokinetic Models
- Predict drug concentrations with any dosage regimen
- Calculate optimum dosage regimen for each individual patient
- Estimate the possible accumulation of drugs and/or metabolites
- Correlate drug concentrations with pharmacologic effect or toxicity
- Evaluate differences in rate or extent of availability between formulations (bioequivalence)
- Describe how changes in physiology or disease affect ADME
- Explain drug interactions
PBPK
Physiologic Based Pharmacokinetic Models
Physiologic Based Pharmacokinetic Models (PBPK)
- Otherwise known as blood flow or perfusion models
- Based on known anatomic and physiologic data
- Blood flow and tissue drug concentrations
- Actual tissue/organ volume is utilized
- Data is difficult to obtain
Compartment Models: One-Compartment Model (image)
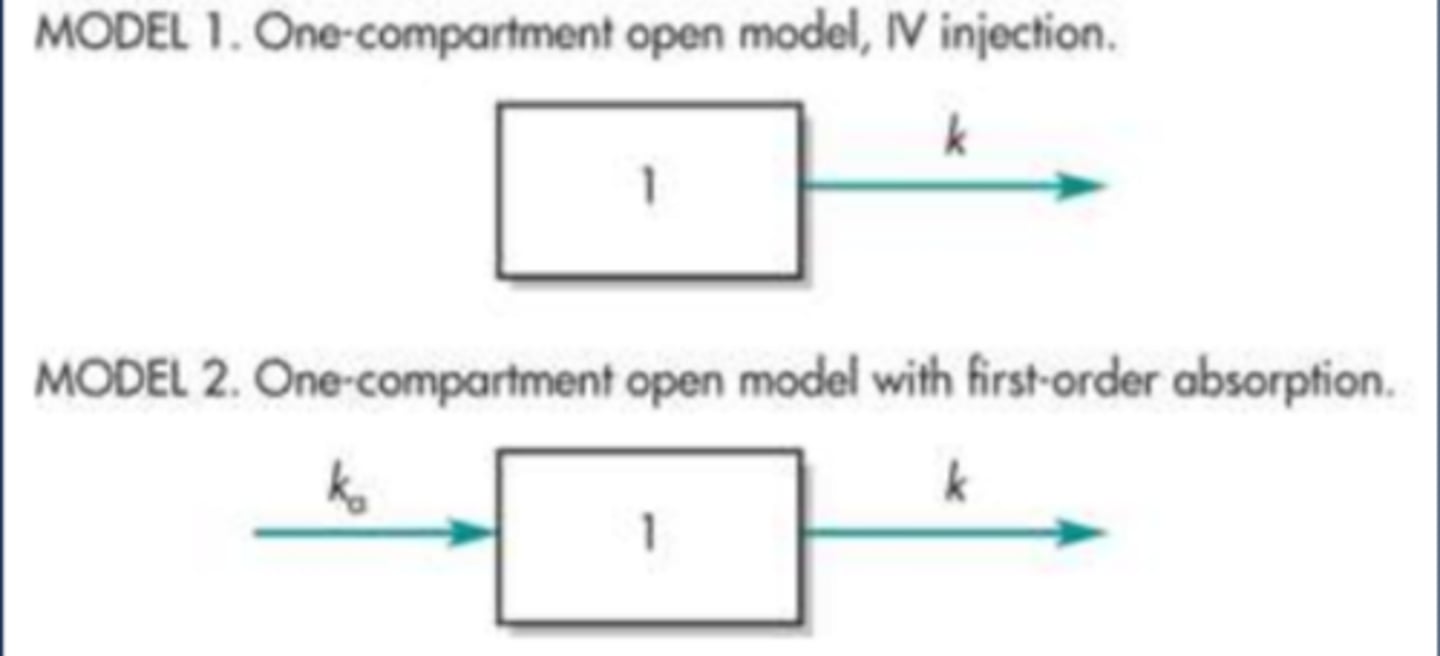
Compartment Models: Two-Compartment (image)
One-Compartment Model Characteristics
- An IV Bolus drug dose is administered directly into the bloodstream, essentially making absorption instantaneous and complete
- The body consists of one single uniform compartment into which the drug instantaneously and rapidly distributes and is then eliminated
- The one-compartment model is a simplistic representation of the processes in the body that determine drug disposition
- The model predicts drug concentrations in the plasma at any point in time, and declines in the plasma concentration will be proportional to declines in tissue concentrations
Two-Compartment Curve Interpretations
- The drug distribution characteristics significantly impact differences observed between one and two-compartment model curves
- The first/central compartment is typically associated with a rapid distribution and equilibration
- The second compartment represents volume into which drug distributes at a slower rate
- The curve can be described and parameters quantified in terms of the distribution and elimination occurring over time = bi-exponential
- Two-compartment models are very complex and require numerous plasma drug concentrations
The equation for the straight line (image)
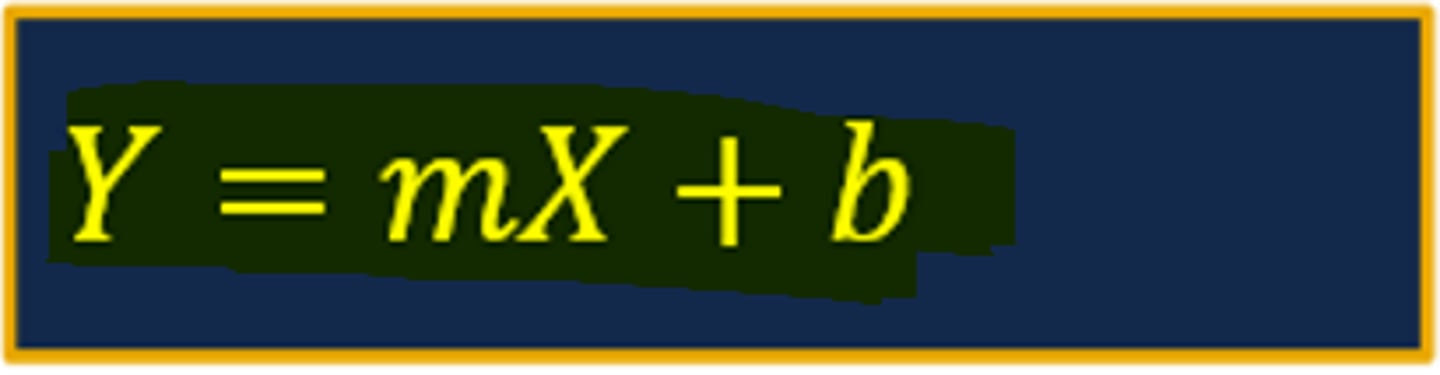
Calculating the slope of the straight line (image)
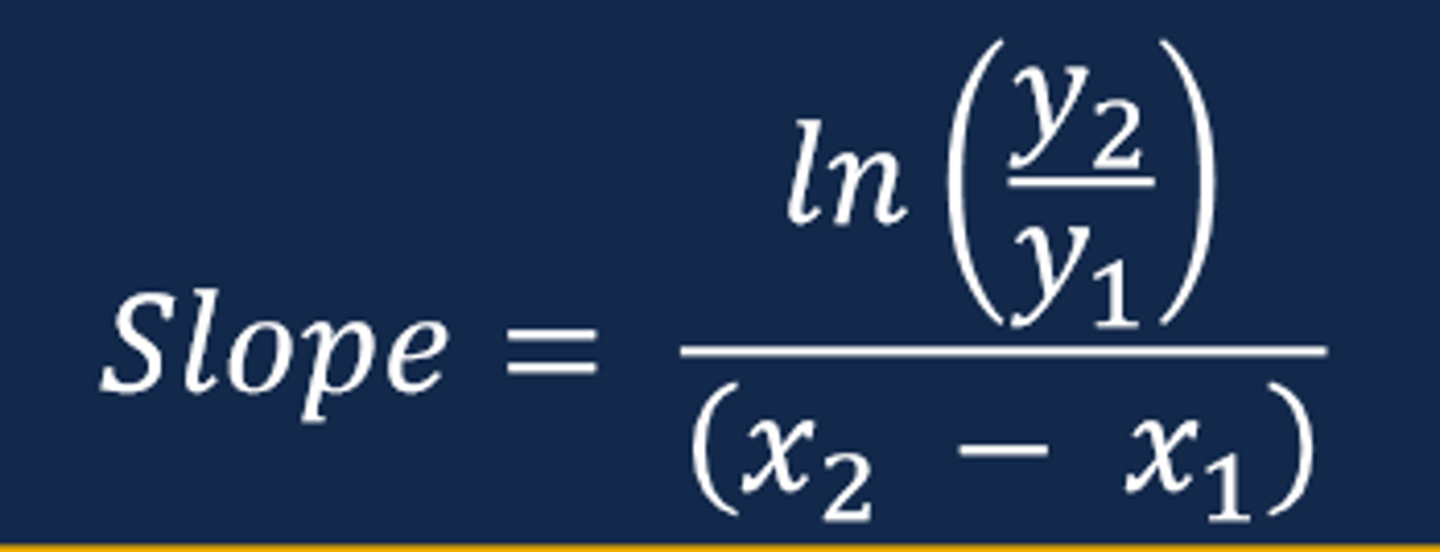
Connecting Slope & Curve to Pharmacokinetic Parameters
- The slope of the straight line from a ln plasma drug concentration versus time curve for a drug displaying first-order elimination will equal the elimination rate constant (ke)
--- Recall that the slope of a ln plasma drug concentration versus time curve is a negative value (negative slope)
--- The steeper the slope of the line indicates a faster rate of elimination compared to a flatter sloped line
- The elimination rate constant (ke) represents the fraction of drug removed from the body per unit of time and is expressed in units of hr-1
Calculating the Elimination Rate Constant (ke) (image)
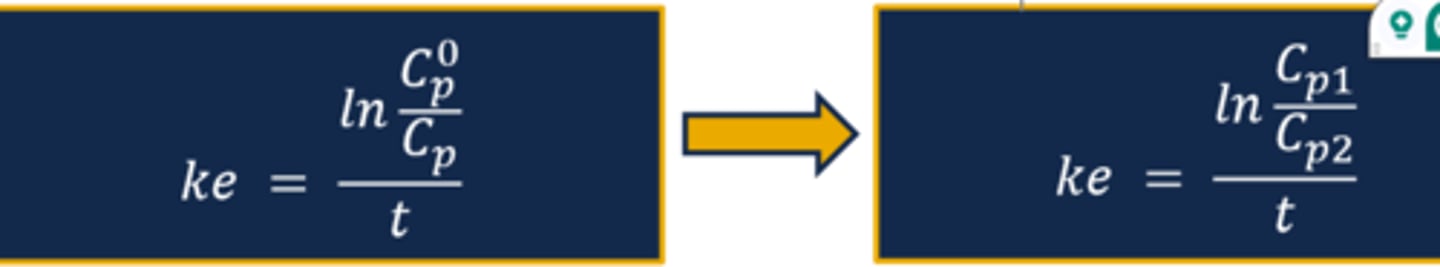
Describing ke Results
- Recall that the elimination rate constant is expressed in units of reciprocal time (hr-1)
- The ke result translates to a fraction or percentage of the drug dose administered that is removed per unit of time
- For example, a ke = 0.15 hr-1 indicates that an initial plasma drug concentration of 10mg/L will decrease by 15% at the end of one hour
- Drugs that follow a first-order elimination process maintain a constant rate of elimination (i.e., elimination rate constant)
- Determination of the ke creates the opportunity to predict the plasma drug concentration at any given time
Drug Half-Life (t1/2)
- The drug elimination half-life represents the time necessary for the drug concentration to decrease by one-half or 50%
- The drug t1/2 provides an indication of drug removal from the body and, ultimately, when an additional dose of the drug is necessary to administer
- The drug t1/2 can be estimated by visual examination of the ln plasma drug concentration versus time curve and mathematically via calculation with the known elimination rate constant
Mathematical Determination of t (image)
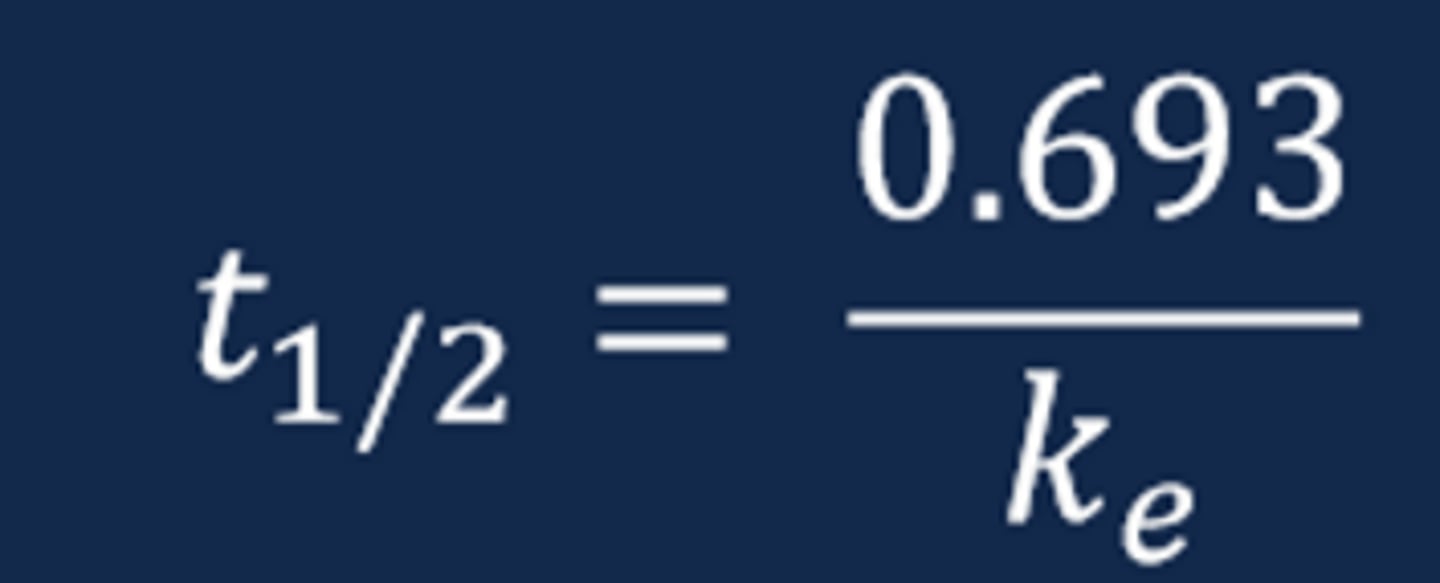
Further Derivation of the Slope Equation
- Utilizing the rules of logarithms and exponents will allow for the further conversion of the straight-line equation
- e-(ke)(t)= the percent or fraction of the initial plasma drug concentration remaining after time "t"
- The equation allows for predictions of drug concentrations in future and the past along the curve
Vd
Apparent Volume of Distribution
Apparent Volume of Distribution (Vd)
- Volume of distribution (Vd) is an indicator of the extent of drug distribution into the body fluids and tissues
- Vd relates the amount of drug in the body to the measured concentration of the drug in plasma (Cp)
- Vd does not represent an actual physiologic compartment in the body
--- Apparent volume of distribution is a theoretical volume into which the drug uniformly distributions immediately following injection into the body
--- Vd does not indicate into which fluids and tissues the drug distributes
Equation for calculating volume of distribution (image)

Vd parameter units for calculation (image)

Additional Maximum Concentration Considerations
- The initial plasma drug concentration (Cp0) also represents the maximum drug concentration or Cmax or C peak
- Calculating the Cmax following an IV bolus dose can be accomplished using the equation below
- The unknown parameter may be provided by patient data or population data
--- The patient concentration may be collected
--- The Vd population value may be observed from population data via an appropriate reference (expressed in L or L/kg)
- Calculating the Cmax provides the maximum concentration obtained for a given Vd following a particular dose
AUC
Area Under the Curve
The AUC is determined by what?
the dose administered and drug clearance, therefore; when clearance remains constant the AUC is directly proportional to the dose administered
The area under the plasma drug concentration versus time curve can be determined utilizing what?
a calculation involving the summation of individual areas of the curve via the trapezoidal rule
The calculation of the AUC is conducted using what?
the observed linear plasma drug concentration versus time curve
The greater the number of known concentrations for use in the calculation increases the what of the calculation?
Accuracy
If the time between the measurements is small (i.e., small width of each trapezoid) then the accuracy is...
greater
Calculating the AUC
- To calculate the AUC, one must calculate the area for each individual area under the curve and sum the areas together
- To create the trapezoid, draw a line vertically to the x axis from each measured concentration
- Then, draw a straight line between the adjacent concentration to identify all of the variables needed for the area calculation
Calculating the estimated AUC for all measured concentrations (image)
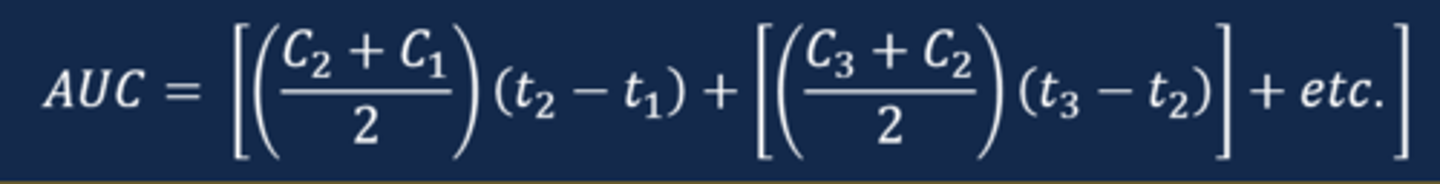
Determination of the terminal portion of the AUC (image)

Cl
Clearance
Drug clearance represents what?
the body's intrinsic ability to remove drug from the blood or plasma
Removal of drug from a volume of blood or plasma in a given time and is expressed as what?
a volume per unit of time (e.g., L/hr)
Clearance does not indicate the amount of drug, but rather what?
the theoretical volume of blood or plasma from which drug is completely removed/cleared in a given period of time
The amount of drug removed depend what?
the plasma drug concentration and the clearance
Concentration rise following bolus (Cmax or Cpeak) (image)

Elimination rate constant (ke) (image)
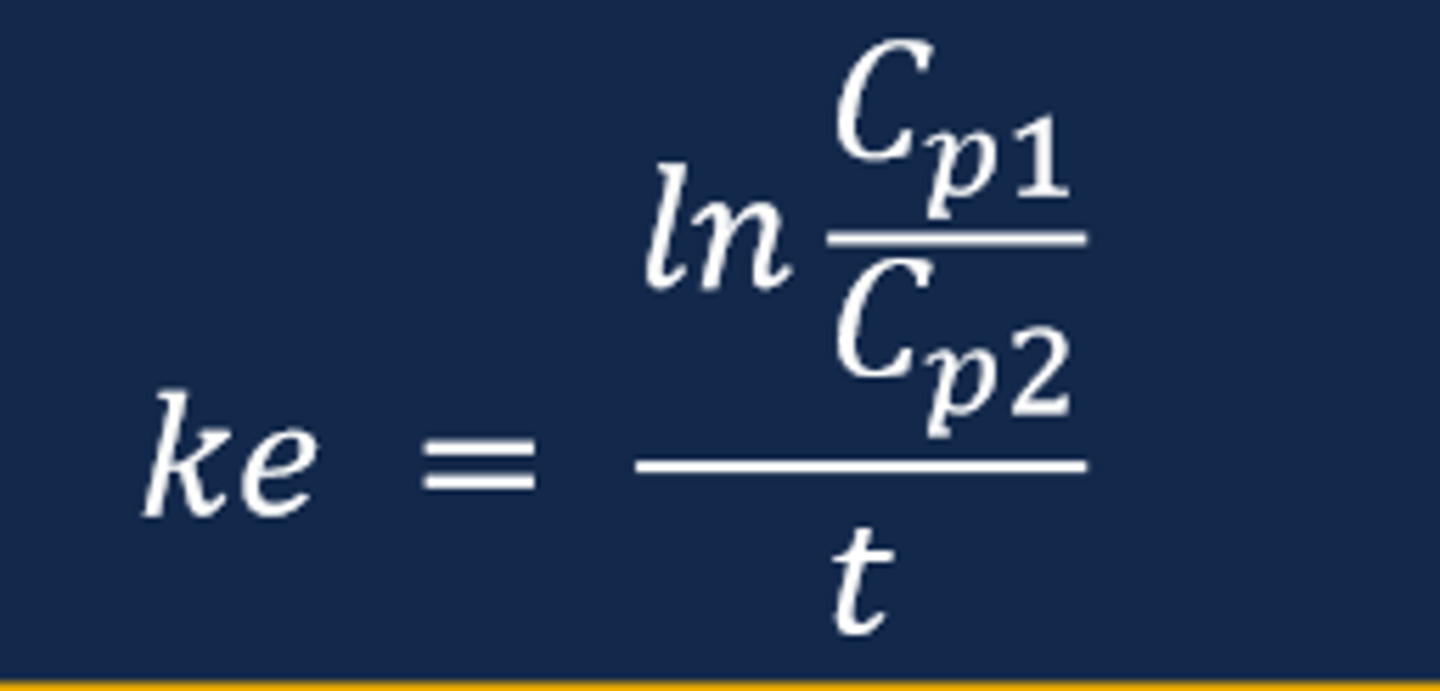
Half-life (t1/2) (image)
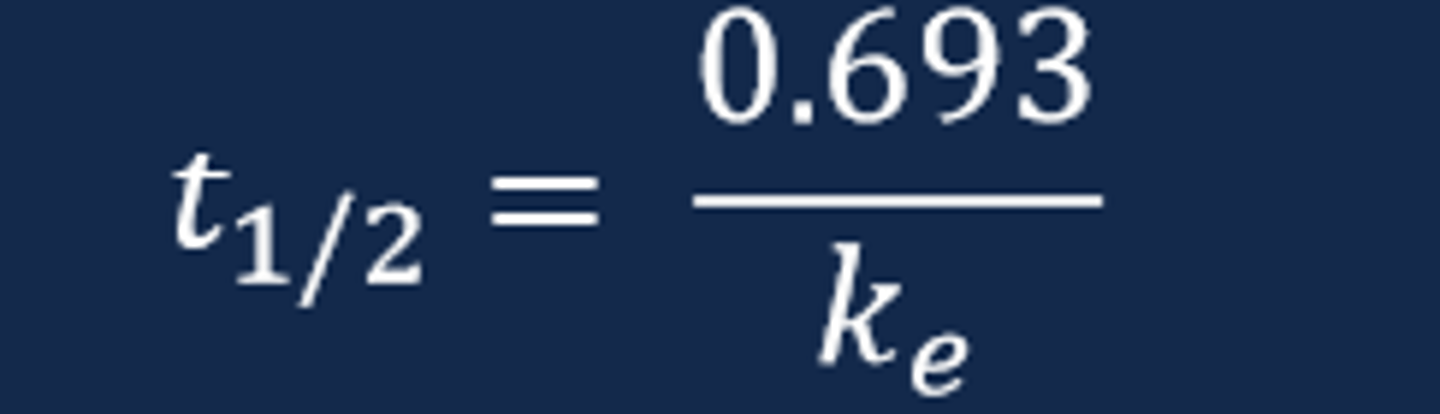
Plasma drug concentration at time "t" along the curve (image)

Pharmacokinetic Processes Overview (image)
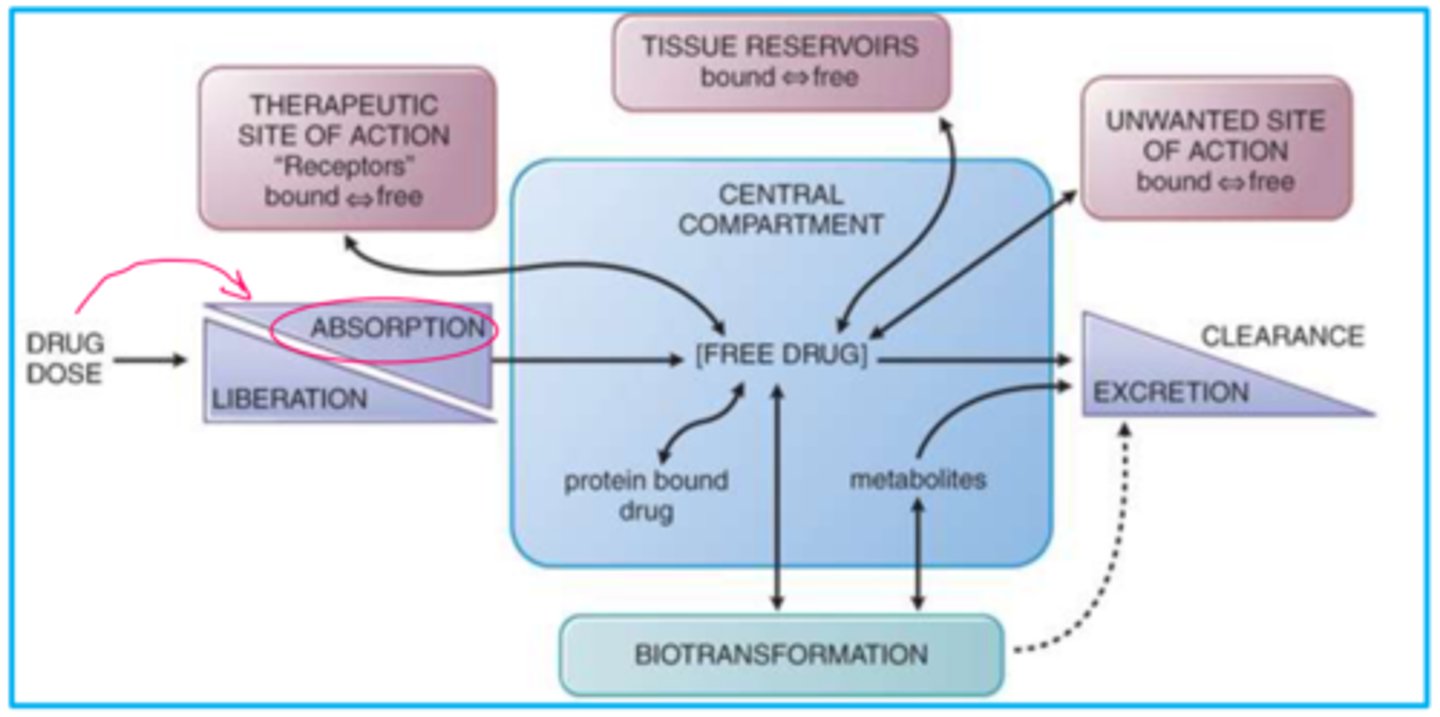
If a drug is intended for systemic activity, then...
the drug must move into the systemic circulation from the site of administration
Absorption, distribution, and elimination of all drugs involves what?
the movement/passage of drug molecules across cell membranes
Movement of drug is impacted by what?
numerous factors including physiochemical drug properties, anatomy and physiological factors of the patient, and the presence of any pathophysiological condition(s)
The Path of the Oral Drug Dose to the Systemic Circulation
1. Oral drug administration
2. Disintegration and drug dissolution
3. Gastrointestinal drug permeation
4. Drug in the portal vein
5. Drug in hepatic vein in the liver
6. Drug in systemic circulation
F
Bioavailability
Bioavailability (F)
the fraction of the unchanged drug dose that reaches the systemic circulation following drug administration from any route
The AUC of the plasma drug concentration versus time curve is reflective of what?
the total amount of drug dose reaching systemic circulation and is proportional to the dose and the extent of bioavailability for first-order elimination drugs
(F) Equation

Methods of Permeation & Transport
- Passive Diffusion
- Carrier-Mediated Transport: Facilitated Diffusion
- Carrier-Mediated Transport: Active Transport