Vaccines & Biosecurity
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
19 Terms
What core principles should guide vaccination decisions in horses?
Risk of exposure to infectious agents
Consequences of disease
Morbidity
Mortality
Zoonotic potential
Potential for adverse reactions
Cost-benefit analysis
Cost of immunization vs cost of disease
Vaccination reduces risk, but does not guarantee protection
Vaccines require time to induce immunity
Often need an initial priming series
Protection varies between horses and over time
Vaccination benefits:
Individual immunity
Herd immunity
What factors influence infectious disease control and spread in horse populations?
Core goals of disease control programs:
Place infected horse in isolation
Reduce exposure to pathogens
Minimize factors that decrease resistance
Enhance resistance through vaccination
Disease risk increases with:
High population density (boarding barns, breeding farms, racetracks)
Movement of horses on/off facilities
Mixing horses from different origins and age groups
Stressors that increase susceptibility:
Stress
Overcrowding
Parasitism
Poor nutrition
Inadequate sanitation
Additional risk factors:
Contaminated water sources
Concurrent disease
Poor rodent, bird, and insect control
Mechanical transfer via people, vehicles, equipment
What are best practices for vaccine use at equine facilities?
Maintain accurate vaccination records
Establish facility-wide vaccination policies
More effective than horse-by-horse decisions
Preserve vaccine efficacy through:
Proper storage
Proper handling
Proper administration and use
What do vaccine label claims mean?
Claims must be data-supported
USDA protection categories include:
Prevention of infection
Prevention of disease
Aid in disease prevention
Aid in disease control (↓ severity, duration, or delayed onset)
Some vaccines claim reduced pathogen shedding
How are veterinary biologics regulated in Canada?
Regulated by the Canadian Centre for Veterinary Biologics (CCVB) under the Health of Animals Act
Oversight includes:
Manufacturing and testing
Labelling
Import/export
Distribution and use
Adverse reaction monitoring
Veterinary biologics include:
Vaccines
Plasma
Tetanus antitoxin
EIA cELISA test kits
Immunomodulators (e.g., Immunocidin, EqStim)
What are characteristics of killed (inactivated) vaccines?
Contain inactivated pathogens (phenol, formalin, β-propiolactone)
Non-pathogenic
Require:
Multiple initial doses
Regular boosters
Adjuvants are critical
Enhance and modulate immune response
Types include:
Whole killed organisms
Protein/subunit vaccines
How do live and modified-live vaccines differ from killed vaccines?
Attenuated but capable of replication
Induce broader and longer-lasting immunity
Stimulate:
Surface (mucosal) immunity
Cytotoxic T-cell responses
Produced by:
Cell culture attenuation
Cross-species variants
Temperature-sensitive mutants
What are recombinant vaccines?
Use genetic engineering rather than whole organisms
Types include:
Live attenuated vector vaccines
Chimeric vaccines
DNA vaccines
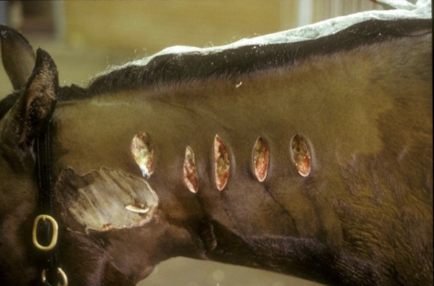
What adverse reactions can occur following vaccination in horses and how are they managed?
Common reactions:
Local swelling and soreness
Fever
Anorexia
Lethargy
Severe reactions:
Aseptic or septic inflammation
Urticaria
Purpura hemorrhagica
Anaphylaxis
Management strategies:
Short-term vs long-term planning
Staging vaccines
Using monovalent vs polyvalent vaccines in sensitive horses

What is the role of epinephrine in vaccine reactions?
Used for anaphylaxis
Common concentration: 1:1000 (1 mg/mL)
Often diluted before use
Routes of administration:
IV
IM
Intratracheal (IT)
How are equine vaccination programs structured?
Divided into:
Core vaccines
Risk-based vaccines
Guidelines are recommendations, not mandates
Veterinarians must consider:
Individual horse risk
Facility management
Product availability
Professional judgment
USDA-licensed vaccines form the basis of guidelines
Use in non-horse equids is discretionary
Which vaccines are considered core for all horses?
Tetanus → 3 boosters before 1year of age
Rabies → yearly booster
EEE/WEE → yearly killed vx given in the spring in canada, water climate 2x/year
West Nile Virus (WNV)
What vaccines are considered risk-based in horses?
Influenza
Equine herpesvirus
Potomac Horse Fever
Strangles
Botulism
Equine viral arteritis
Rotavirus
Anthrax
Others:
Snake bite
Venezuelan Equine Encephalitis (VEE)
What newer or future vaccines are being explored in horses?
Rhinitis virus
Leptospirosis
Lawsonia intracellularis
Lyme disease
Rhodococcus equi
What is the foundation of equine biosecurity?
Anything contacting a horse can transmit pathogens
High-risk materials include:
Manure, urine
Nasal, ocular, vulvar secretions
Fetal fluids and tissues
Abscess drainage
Humans can act as vectors
How should personnel and protective equipment be managed during outbreaks?
Ideal: separate staff for sick vs healthy horses
Practical order of care:
Healthy animals
Exposed animals
Sick animals
Use of PPE:
Gloves
Gowns/coveralls
Shoe covers
Eye protection
Proper disposal of contaminated materials
What hygiene and containment measures support biosecurity?
Hand hygiene:
Soap and water ≥15 seconds
Alcohol-based sanitizers (≥62% ethanol)
Animal containment:
Controlled movement
Clear boundaries and signage
Footbaths and wash stations
Footbaths:
May be inactivated by organic matter
Less effective in cold temperatures
Must be safe if contacted or ingested
How should facilities manage environmental contamination?
Solid stall barriers to reduce aerosol spread
Avoid shared equipment and water sources
Proper manure and bedding disposal
Separate equipment for contaminated areas
Cleaning protocol:
Rinse → wash → rinse → disinfect → rinse → dry
Disinfectant categories include:
Alcohols
Chlorhexidine
Halogens
Oxidizing agents (Virkon-S)
Phenols
Quaternary ammonium compounds
Why must non-horse animals be considered in biosecurity plans?
Dogs, cats, rodents, and birds can act as reservoirs or mechanical vectors
Vermin control is essential to disease prevention