biochem cool 2
1/38
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
39 Terms
oxidative deam of glutamate - localisation, main function,
regulation (main one and then allosteric activators/inhibitors)
OX DEAM
mitochondria
main function = only deg of AA where energy is obtained
glutamate dehydrogenase
allosteric act = ADP,
allosteric inhibitors = ATP and GTP

oxidative deam of remaining AAs
enzymes, what do these enzymes act together with
what is the released H2 transferred to
REMAINING = GIMME NOW = IMINO

transamination
what interacts with what?
the start - is converted into a new what? (two things)
cofactor of transaminase?
role ?
AA (donor of amino group) interacts with acceptor of amino group (keto acid)
keto into new l amino acid and vice cersa
cofactor = PLP (pyrodixal phosphate - derivative of b6)
role = to make NON ESSENTIAL amino acids

AST GOT and ALT GPT
what are they?
used for what disease? how do they work?
what other disease?
draw aspartate
aspartate = COO- at the end
ast/alt = TRANSAMINASES WITH BIO IMPORTANCE
DIAGNOSTIC MARKER FOR LIVER DISEASE (inc proportionately depending on disorfer)
myocardial infarction = increase in AST serum.

transdeamination:
what two things is it a combination of - what enzyme does it use?
what is it the main route of?
what does it allow AA’s to have done to them?
which direction is it in? leading to the synthesis of?
cmobo of transamintion of AA with a aloha ketoglutarate and subsequent oxidative deamination of the resulting glutamate
under glyutamate dehydrogenase
main route for nitrogen release from AA’s
a way to degrade the other AA’s with energy gain
in the refuction direction = synthesis of non essential AAs
decarboxylation
WHAT ARE BIOGENIC AMINES NEUTRALISED BY
what are biogenic amines
what are they neutralised by
mono amine oxidases, di amine oxidases

reductive animation of ketoglut
WHICH ENZYME IS INVOLVED
what is it and what does it cause?
reduces the level of what required for what?
reverse ox deam of glut -
high level of glutam dehydrogenase in the brain
reduces amount of aKG REQUIRED!!!!! FOR CITRATE and glutamate synthesis

glutamine synthesis
requires ATP
causes high levels of glutamine synthetase in the brain
reduces level of glutamate which is a neurotransmitter required for synthesis of FABA
glutamine is used for ammonia depot = degraded by glutaminase

ammoneogenesis 9METABOLISM OF GLUTAMINE)
location, main function (3 points)
what is obtained and what is it used for?
KIDNEYS
neutralisaiton of ammonia
-obtained from degredation of glutamine
by its conversion into ammonium ion and excretion through urine
glutamate obtained from deg of glutamine is used as a substrate for GNG

urea cycle
localisation
main function
regulation - main regulatory enzyme, the two allosteric activators
energy balance?
enzyme defects
4 macroergic bonds for 2 AA groups - ammonia and aspartate and bicarbonate ion to urea
enzyme defects = ammonia posion = vomiting, mental retard

synthesis of SAM
main function? what is SAM a cofactor in
SAM is active form of?
what happens in methionine deficiency?
what is needed as a cofactor?
what parts do you forget of the scheme
atp and pPP on meth ad. transf. serine, glycine, hsitide, tryptophan goes to c1 group

degradation of phenylalanine and tyrosine
products:
enzyme defects: 4
parts of scheme you forget
phenylketonuria
tyrosinemia
alkaptonuria
albinism
phenylalanine hydroxylase (o2 to H20) (BH4 to BH2)
tyrosine transaminase = akg and glutamate
pOH phenylpyruvate hydroxylase again = O2 to CO2 (Cu2+)

dihydroprotein reductase - draw it
what does it do?
requires what?
defect in this enzyme leads to what?

synthesis by transamination OF A KETO ACIDS- what is obtained?
hint - its the stuff from ast and alt action

synthesis by reductive amination - what is obtained under action of what

synthesis by amidation (making it an AMINE)
what is obtained from twhat (2 things)
under action of what?

synthesis by hydroxylation
hydroxylation of what obtains what
by hydroxylation of phenyl alanine, tyrosine is obtained
ESSENTIAL AA

synthesis by cyclation (of what?) produces what
what does it require
CYCLATION of glutamate gives proline = a cycler is a proathelete
requires nadph for lots of energy

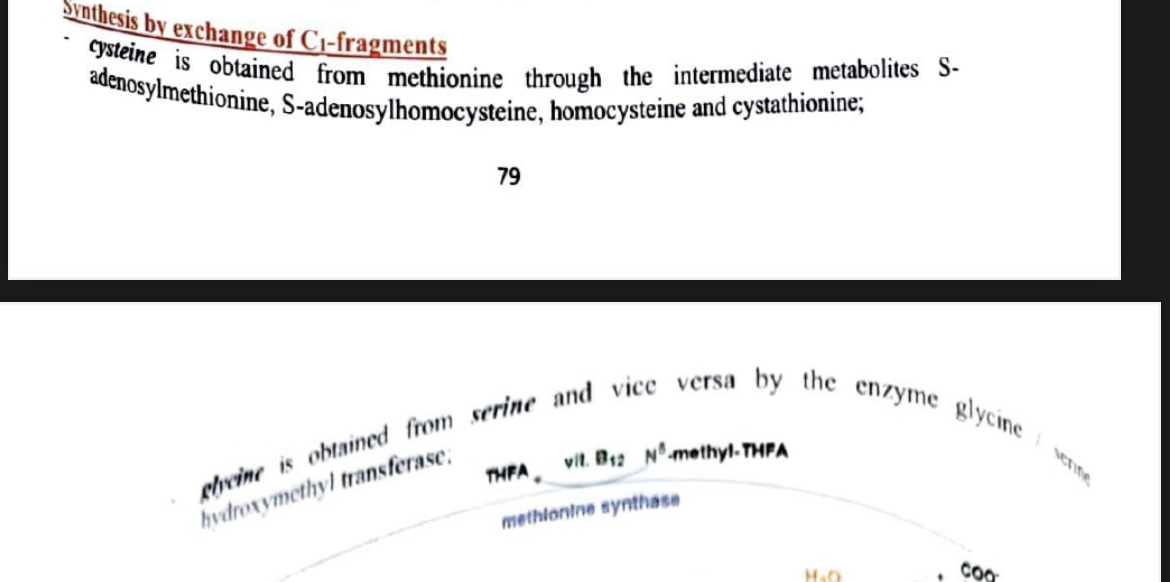
synthesis by exchange of c1 fragments
ontained from what through what ?
c1 fragment = C AND C (from methionine) through methyl transferases
creatine phosphate - forgot
loc !!!!!!!!!!!!!!!!!!!!
main function?
main tip?
aas involved?
things i forget in the scheme:
arginine to guanoacetate - amidino transferase GLYCINE _ ORTHININE
creatine phosphokinase - atp to adp
h20+P
THE LOCATIONS KIDNEY, LIVER, MUSCLE + BRAIN

cituline formation?
what is under the enxyme
structure of citruine
what is not involved therefore what cant happen?
what is it obtained from by what?
what do you froget?
NO also produced = 2nd mediator for vasodilation
NADPH to NADP
citruline = CO=O and NH2

synthesis of acetylcholine
localisation
main function?
AA substrate for synthesis?
cytosol of neruons
formatiion of acetyle choline, a neurotransmitter,
serine
synthesis of serotonin and melatonin? forgot what
TRIP
AA substrate for synthesis of it?
serotonin localisation?
what does it participate in?
what does it activate?
melatonin?
localisation?
obtained from what?
synthesis and what else increases at night? what does it affect?
what does it inhibit?
melatonin/seratonin = hormones that make u trip out
therefore tryptophan
CLEAN THEN DIRTY AT THE TOP
plain 6 pyramid then pentagon with n at the top
catecholamines = tyrone the cat
acetylcholine
sam S A)AETYL) - hes neuro divergent therefore neurons
melatonin = secrete at night - affects circadian rhythm = inhibits other neurotransmitters
(where n acetyltransferase is in pineal gland)
synthesis of catecholamines - what to remember
name the catecholamines:
substrate:
location:
regulation: main regulatory enzyme?
disease?
WHAT DO YOU FORGET?
NEED TO FEED THE CAT WATER
dopamine, adrenaline, noradrenaline
tyrosine
location:
noradrenaline/dopamine = neurotransmitt. in NS
adren/noradren = made in medulla of adrenal gland = for stress
main reg enzyme = tyrosine hydroxylase
parkinsons
DOPAMINE B HYDROXYLAASE
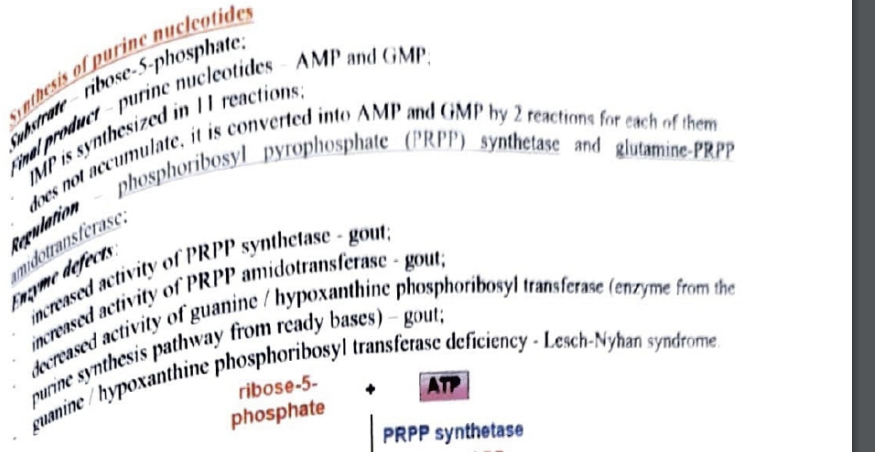
degradation of pruine nucleotides - starts with what preoduct?
location
final profuct?
regulation?
what is a suicide subtrate for the regulatory enzyme? and what is it used for?
eynzme defects?
parts you forget
IF YOURE PURE YOU WANT PROTEIN AND GOUT
adenosine deaminase = h20 to nh4+
guanase = h20 to nh3+

origin of the constituent atoms of the purine ring

synthesis of pruine nucleotides do the CORRECT SCHEME PLEASE
subtrate?
final product?
how many reactions form IMP?
why does it not accumulate?
REGULATION!!! ?
enzyme defects
5
what syndrome is caused and how?
enzymes on the diagram you forget = ?
enzyme defects:
PRPP synthase or PRPP glutamyl amidotransferase increase = gout
hypoxanthine phopsphoribosoyl transferase deficiency = gout
defeciency in this = lesch nyhan syndrome
glutamate to glutamine in glutamyl thingy
and again in transamidinase
and atp to p + pp in prpp synthesis
regulation of purine nycelotide synthesis
part i forget =
what provides energy for synth of AMP and GMP
ADP / GDP = INHIBITORS
main regulatory enzyme?
allosteric activator?
allosteric inhibiotrs?
final products?
name the competitive inhibitors and how they work here
main reg enzyme = glutamyl PRPP amidotransferase
allosteric activator - PRPP
alloesteric inhibitors: - ADP AND GDP
allosteric inhibitors - final products: ATP<AMP>ADP< GDP< GTP< GMP
PRPP SYNTHETASE
AMP AND GMP = competitive inhibitors of iIMP AND EACH INHIBITS THEIR OWN SYNTHESIS
ATP provides energy synth for GMP and GTP for AMP
synthesis of pyrimidine nucleotides - miastkaes
what is built first before the next part is added?
substrates?
final product?
how many reactions is UMP synth in? what is obtained from it?
regulation - main reg enzyme?
enzyme defects?
2
aspartate = c-O- and COO,
no nh for ctp
the base is first built then the ribose phosphate residue is added
substrates: glutamine + bicarbonate
final product: tmp, ctp, ump
6, other pyrmaidines
regulation: aspartate transcarbamoulase
enzyme defects =
orotidine monophosphate decarboxylase and orotate phosphoribosyl transferase = orotaturia = type 1
orotidinedecarboxylisasae = type 2
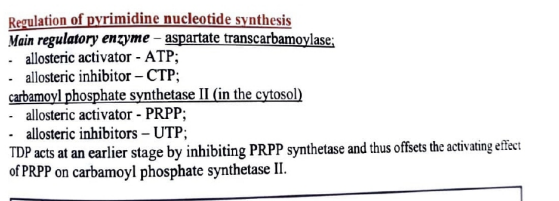
regulation of pyrimidine nucleotide synthesis
main reg enzyme
alloesteric activator
allosteric inhibitor
which enzyme regulaties it in the cytosol? (at the start)
alloesteric activtor and inhibitor?
what does TDP do here?
free iron (non heme) - intake of iron by enterocytes
what do you forget
removal of iron from enterocyte
deposition of iron in liver and release into circulation again
on luminal surface of cells it is reduced from fe3 to fe2 by ferrireductase (duodenal cyt b), transported into enterocyte by divalent metal transporter
iron crosses membrane through ferroportin (crosses basolateral membrane) and hephaestin oxidses it from fe2 to fe3 again
and then fe3+ binds to transferrin and passes into liver + released into circulation again
hepatocytes absorb fe3+ + transferrin after it binds to tfr (transferrin receptor)
fe2 is released into blood and oxidised to fe3+ by ceruloplasmin and binds to transferring again
heme iron - intake of iron by enterocytes
transported by HCP 1 (hemetransporting protein)
iron is released by heme oxygenase and fe2+ binds to ferretin again
porphoryn synthesis
location
main function
substrates
regulation: main reg enzyme
allosteric inhibitor (what else is this substance)?
inductors:
repressors?
enyzyme defects
mainly liver and bone marrow
main fynction: fomation of porphoryns, pros groups, hemeoglbin myoglonin etc
succinyl coA and glycine
main reg enzyme = ALA synthase
allosteric inhib = heme (is also a repressor)
inductorsL anaesthetics, xenobiotics, steroids
repressors: glucose, hemin, hematin
enzyme defectsL
decreased ALA dehydratase activity
porphoryn - mutation in gene 3 and 8
intermittent acute porphyn - iroporphyrinogen 1 synthase
degradation of hemoglobin
localisaiton
main function
macrophages: how is hemohlobim degraded here, what is obtained
in liver?
describe the indirect/direct bilirubin again
macrophages and hepatocytes in the liver
main funct: erythrocutes live for 120 days - after this they degrade
globin = into AA (reused)
heme = bilirubin )and iron is released which is recycled)
MACROPHAFES:
under heme oxygenase, green biliverdin is produced
indirect bilirubin is produced (conjugated, blood, hydrophobic)
in liver, it is converted to bilirubin diglucoronide after it is conjugated with udp glucoronic acid to form bilirubin diglucoronide (bile, hydrophilic, direct)
metabolism of bilirubin
in macrophage, heme is converted to indirect bilirubin
is bound to albumin, transported to liver by blood
indirect bilirubin = taken up by parenchymal cells, conjugated to direct bilirubin and secreted into bile
in intestines, bilirubin is reduced to urobillonogen
in colon, it is oxidised to urobilins (gives poo normal colour)
some of urobilinogen enter kidneys and exit via blood stream, while some are reabsorbed by intestines, enter liver and re exreted (enterohepatic circulation)
haemolytic jaundice
reason?
degradation of large amounts of erythrocytes exceeding the capacity of a healthy liver
bilirubin in the blood increased (indirect)
no bilirubin in urine
urobillnogen IS, as dark as beer urine, DARK feces
hepatic jaundice
hepatocytes = destroyed due to liver damage (cirrohosis/hepatitis)
bilirubin in the bloos - yes (increased indirect and direct)
bilirubin in urine = yes
urobillinogen = not increased
feces colour = normal
obstructive (post hepatic jaundice)
disorder name?
reason - due to complete obstruction of bile duct due to tumour / gall stones
bilirubin in the blood = increased direct bilirubun
bilirubin in urine = yes, dark urine
urobilinogen in urine, no
feces color = acholic (pale)
cysteine synthesis