5: Stoichiometry & Concentration of Solutions
1/60
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
61 Terms
StoichiometryGreek word “Stoicheion” which means elements and “metron” which means measure
branch of Chemistry that deals with the mass relationships of elements in compounds and the mass relationship between reactants and products in chemical reactions
elements; measure
Greek words:
“Stoicheion” which means _________;
“metron” which means _________a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data.
Stoichiometry
a section of chemistry that involves using relationships between reactants and/or products in a chemical reaction to determine desired quantitative data.
mole
→ It is the SI unit for the amount of substance. → It is the amount of substance that contains the same number of particles as the number of atoms in exactly 12g of Carbon-12
Amadeo Avogadro
Avogadro’s number was named after ______________________
Avogadro’s Number
→ it is the number of particles in exactly one mole of a pure substance.
→ Exactly 12 g of Carbon -12 atoms contains an Avogadro’s number.
6.022 x 1023
value of Avogadro’s Number
Molar Mass
→ It is the mass in grams of one mole of an element.
→ The mass of one mole of atoms in grams, is numerically equal to the atomic mass of the element in atomic mass unit.
Empirical Formula
– it gives the simplest formula of a compound.
REACTION FORMULA
MASS-MOLE STOICHIOMETRY
2 types of Stoichiometry
mole ratio
is the conversion factor that relates the number of moles of any two substances involved in a chemical reaction.
reactants
The substances that go into a chemical reaction are called the
products
the substances produced at the end of the reaction are known as the
Limiting Reactant
The reactant that is completely used up in a chemical reaction. It determines the amounts of products formed.
Excess Reactant
A reactant that is not completely used up in a chemical reaction
Actual Yield
It is the amount of product formed from the actual chemical reaction and is usually less than the theoretical yield.
Theoretical Yield
It is the maximum amounts of products which could be produced by the complete reaction of limiting reactant.
Percent Yield
It is used to express the efficiency of the reaction
= Actual Yield / Theoretical Yield x 100%
MOLARITY (M or g/L)
tells us the number of moles of solute in exactly one liter of a solution
MOLALITY (m or mol/kg)
tells us the number of moles of solute dissolved in exactly one kilogram of solvent.
MOLE FRACTION (X)
he ratio of the number of moles of that component to the total number of moles of all components in the solution.
Normality
is the concentration expressed as the number of gram equivalent weights (or number of equivalents, abbreviated equiv) of solute per liter of solution.
equivalent weight
the weight of a substance that will react with, combine with, contain, replace, or in any other way be equivalent to, one mole of hydrogen atom or hydrogen ion or hydroxide ion
Law of Conservation of Mas
→ The total matter in the reactants of any chemical reaction is equal to the total quantity of material in the product.
→ It asserts that the overall mass of the substances reacting in a balanced manner remains unchanged consequently when original chemicals are transformed into novel materials
limiting reactant
The reactant that is used up first is known and is the one that has been used up entirely
excess reactant
the reactant that remains after a complete reaction
theoretical yield
the quantity of product generated that would remain after the reaction has consumed all of the limiting reactant.
→ The highest amount of the product that may be produced from a specific amount of limiting reactant
actual yield
which is typically less than the theoretical yield, is the amount of product that the reaction actually produces
aqueous solution
a solution in which the solvent is water. In such solutions, various substances, called solutes, are dissolved in water.
Aqueous reactions
chemical reactions that occur in water as the solvent.
Precipitation reaction
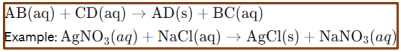
Precipitation Reactions
in these reactions, insoluble solid compounds, called precipitates, are formed when two aqueous solutions are mixed.
Acid-Base Reactions (Neutralization Reactions)
These reactions involve the transfer of protons (H⁺ ions) from an acid to a base to form water and a salt
Acid-Base Reactions (Neutralization Reactions)
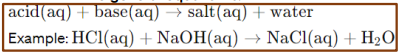
Strong Acid-Strong Base Neutralization
In this type of reaction, a strong acid reacts with a strong base to form a salt and water
Weak Acid-Strong Base Neutralization
: Here, a weak acid reacts with a strong base to form the salt of the weak acid and water.
Strong Acid-Weak Base Neutralization
This type involves a strong acid reacting with a weak base to form the salt of the strong acid and water.
Weak Acid-Weak Base Neutralization
In this scenario, both the acid and base are weak. The products include the salt formed from the cation of the base and the anion of the acid, along with water.
Redox Reactions (Oxidation-Reduction Reactions)
These reactions involve the transfer of electrons between reactants.
Combustion Reactions:
Displacement Reactions
2 types of Redox Reaction
Combustion Reactions
A substance reacts with oxygen gas to produce heat, light, and often, water and carbon dioxide.
Combustion Reactions
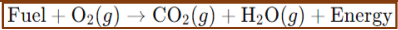
Displacement Reactions
A more reactive element displaces a less reactive element from its compound.
Displacement Reactions
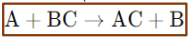
Complexation Reactions
In these reactions, a complex ion is formed when a central metal ion combines with one or more ligands (molecules or ions that donate electrons to the metal ion
Hydrolysis Reactions
These reactions involve the breakdown of a compound by water. They are often observed in the reactions of salts with water to produce acidic or basic solutions.
Hydrolysis Reactions
Strong Acids
completely ionize in water, meaning they dissociate almost entirely into ions
s include hydrochloric acid (HCl), sulfuric acid (H₂SO₄), nitric acid (HNO₃), hydrobromic acid (HBr), hydroiodic acid (HI), perchloric acid (HClO₄), and chloric acid (HClO₃).
have high conductivity in aqueous solution due to the abundance of ions.
typically have low pH values (less than 3) in aqueous solution
Weak Acids
partially ionize in water, meaning only a small fraction of the acid molecules dissociate into ions.
include acetic acid (CH₃COOH), formic acid (HCOOH), carbonic acid (H₂CO₃), and many organic acids.
have lower conductivity compared to strong acids because of the lower concentration of ions.
usually have pH values greater than 3 in aqueous solution
Strong Bases
fully dissociate in water, yielding a high concentration of hydroxide ions (OH−).
include sodium hydroxide (NaOH), potassium hydroxide (KOH), lithium hydroxide (LiOH), and calcium hydroxide (Ca(OH)₂).
have high conductivity in aqueous solution due to the abundance of hydroxide ions.
have pH values greater than 11 in aqueous solution.
Weak Bases
partially ionize in water, resulting in a lower concentration of hydroxide ions.
include ammonia (3NH3), methylamine (CH3NH2), and other organic amines.
have lower conductivity compared to strong bases because of the lower concentration of hydroxide ions.
usually have pH values less than 11 in aqueous solution.
Balanced Equations:
Balanced equations represent chemical reactions where the number of atoms of each element is the same on both sides of the equation. This ensures the law of conservation of mass is obeyed.
Complete Ionic Equations
Complete ionic equations show all ions participating in the reaction, including spectator ions that do not undergo any change.
Net Ionic Equations
Net ionic equations focus only on the ions that participate in the reaction, omitting spectator ions
Redox Equations
Redox equations represent chemical reactions involving oxidation-reduction processes, where electrons are transferred between reactants.
Half-Reaction Equations
Half-reaction equations represent either the oxidation or reduction part of a redox reaction, showing only the electron transfer
H⁺
Neutralization in acidic solutions, you add ___ ions directly
OH⁻
In basic solutions, you add ____ ions to neutralize any excess H⁺ ions present in the equation.
water molecules (H₂O)
These additional OH⁻ ions then react with H⁺ ions to form _________ in the balanced equation.