intro to amines and base strength
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
what is an amine?
compounds based on ammonia where H atoms have been replaced by alkyl or aryl groups
what is an aliphatic amine?
amines with alkyl substituents
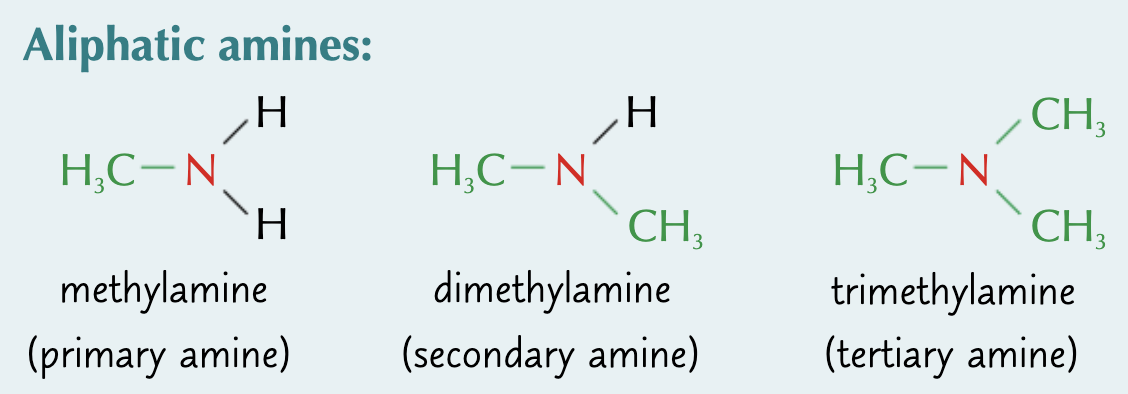
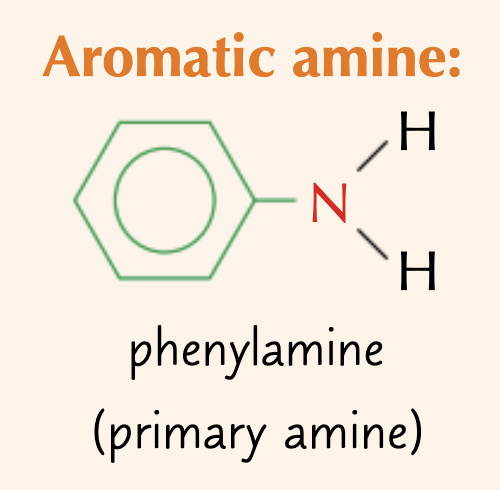
what is an aromatic amine?
amines with aryl substituents
describe the features of an quaternary ammonium salt:
not an amine!
+vely charged N atom bonded to 4 alkyl groups
has a counter ion (to balance charge)
formed when lone pair on 3o amine bonds with a 4th alkyl group
how do we name amines?
usually use “amine” as a suffix (e.g. propylamine)
but sometimes use “amino” as a prefix e.g. 1-aminopropane
what is a base?
proton acceptor
are amines strong or weak bases? why?
weak bases - lone pair on N atom can accept a H+
ability of ammonia/amine to act as a base depends on how well the N’s lone pair can accept a proton
the higher the e- density on the N, the more easily it will accept a proton
describe and explain the pattern for base strength in amines/ammonia:
aromatic amine < NH3 < 1o < 2o < 3o
the more alkyl groups, the more available the lp on the N atom is
so there is a greater +ve inductive effect
why is the base strength of an aromatic amine low?
N lone pair partially delocalised into the benzene ring, decreasing base strength