Unit 4: Nuclear Chemistry
1/92
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
93 Terms
Radioactive
Generally caused when an atom is unstable
Nuclear stability
The larger (more massive) a nucleus is, the harder it is for it to stay together
Natural decay
When a nucleus is radioactive, it gives off decay particles and changes from one element to another
Other words for natural decay
natural transmutation
Band of stability
the location of stable nuclei on a neutron-vs.-proton plot
What atoms have at least one stable isotope
Atoms with an atomic number of 1 through 83
What atoms are more reactive
All isotopes of elements about 84-natural radioisotopes
Nuclei above the belt have
too many neutrons, stabilized by beta emission
Beta emission
emits electron
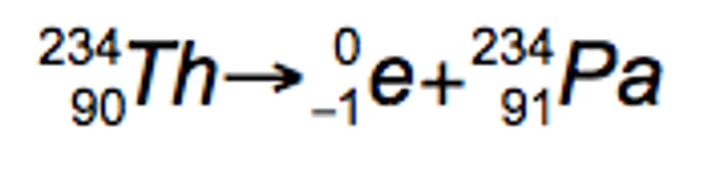
Nuclei below the belt have
too many protons, positron emission/electron capture
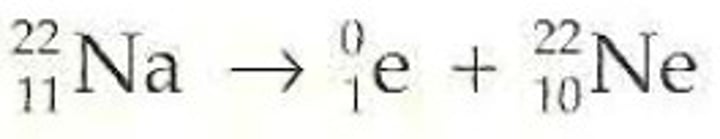
Nuclei that have p>84
both too many protons and neutrons-a-emission
A emission
Alpha-He 2/4
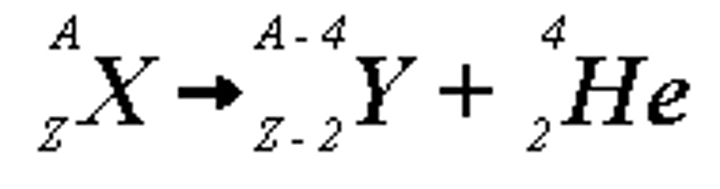
Natural radioactivity
Spontaneous disintegration of the nucleus of an atom with the emission of particles or energy
Modes of decay
Alpha, beta, gamma, neutrons
Alpha decay; symbol, charge, mass, penetration strength
a, 2, 4, Low (apple)
Beta decay; symbol, charge, mass, penetration strength
B, -1, 0, moderate
Gamma decay; symbol, charge, mass, penetration strength
y, 0, 0, High
Neutrons decay; symbol, charge, mass, penetration strength
n, 0, 1, moderate
Radioactive materials will continue to decay until
they reach a stable # (usually with atomic # less than 83)
Beta particles
Fast moving electrons-little mass, stops ~1 cm into body
In Beta Decay, the effect is that
a neutron is converted into a proton, ejecting an electron from the nucleus
There are no electrons
in the nucleus. The ejected electron is formed when energy released from the nucleus 'congeals' into mass (E=mc^2)
Gamma radiation
Consists of high-energy photons, can penetrate to internal organs
Gamma ray symbol
0/0 y
Gamma rays are emitted when
nucleons rearrange into a more stable configuration
Gamma rays often
accompanies other nuclear decays and DOES NOT change the identity of the nucleus on its own
Positron decay
Images
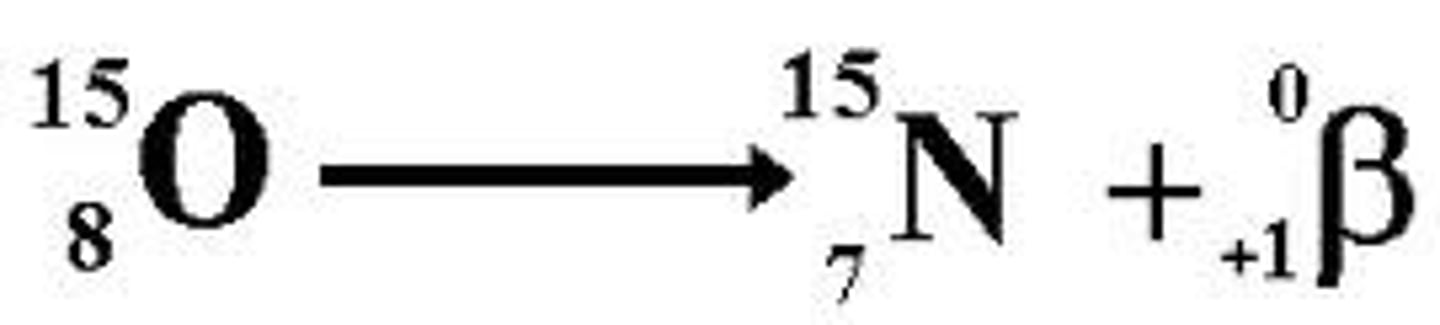
Positron
identical to an electron, except that it has a positive charge
Neutrino
massless, chargeless particle
Electron capture
Nucleus captures orbiting electron
Positron decay image
Imagee
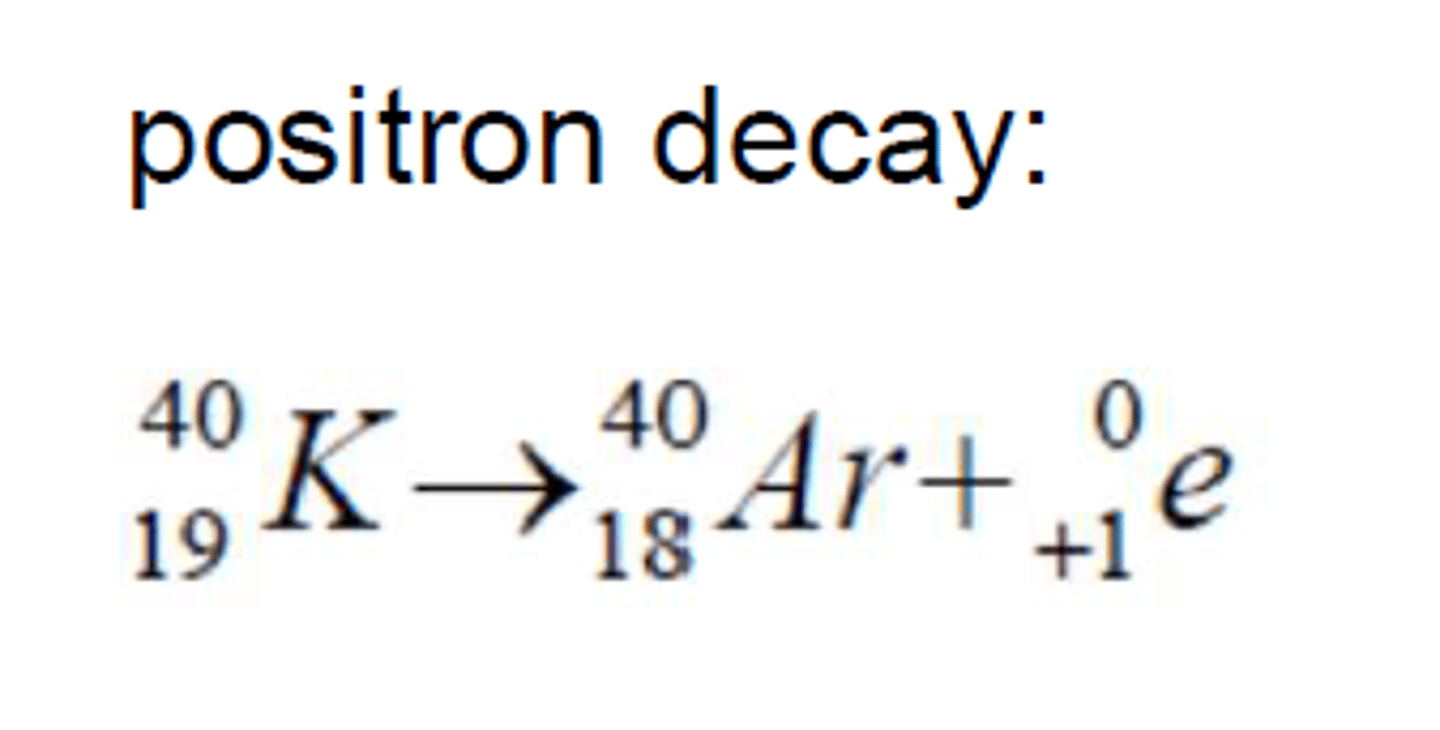
Electron Capture image
Imagee
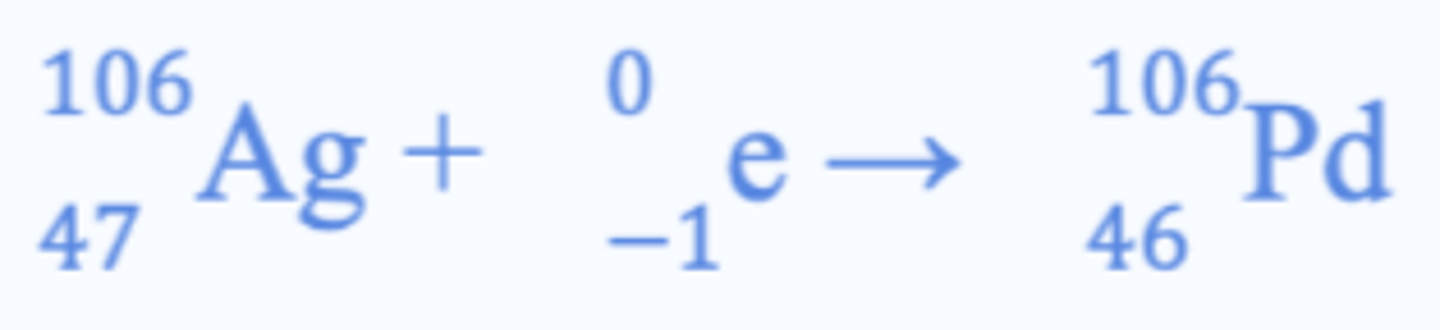
Transmutations
When a nucleus decays into a new and different nucleus (also called radioactive)
Penetration
How far into a material the radioactive particle will go
Physical Reaction-same
compound and mass, charge
Physical Reaction different
Phases (s->l)
Chemical Reaction same
Mass and number of same atoms, charge
Chemical Reactions different
Compounds
Nuclear Reaction same
mass and charge
Nuclear reaction different
Elements
Rate of radioactive decay
each radioisotope has a unique rate of decay-half-life
An isotope's half life is
independent of temp, pressure, and its state of chem combination
useful in radioactive dating
Half-life
The period of time that must go by for half of the nuclei in the sample to undergo decay
During a half-life period
half of the radioactive nuclei in a sample decays to a new, more stable nuclei
How to find half life
1.) divide total time by one half life, which is # of half lives
2.) Count arrows
artificial transmutation
"man made" reaction caused by hitting a nucleus with a high energy particle, such as a neutron or alpha particle
Why do you need high speed particles in artificial transmutation
Alpha particle and nucleus are both positive, so they will repel each other otherwise
Unique to natural decay
Single unstable reactant decays into a decay particle and new, more stable nucleus
Common to natural transmutation and artificial transmutatiob
Mass and charge are conserves, both form new elements and produce energy
Unique to artificial transmutation
Stable nucleus and particle bullet collide to produce new products
Both natural transmutation and artificial transmutation produce
energy but natural decay produces less
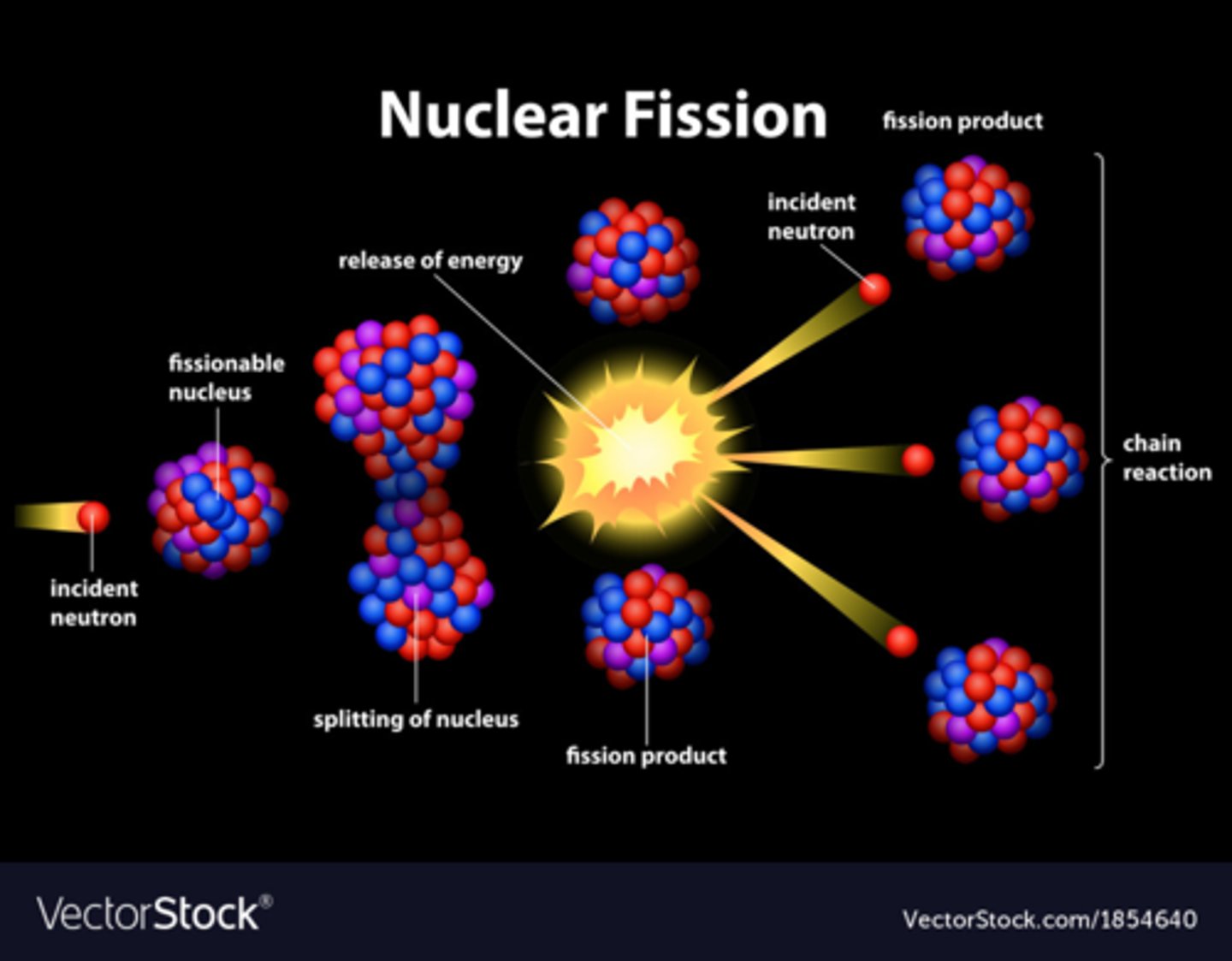
Fission
Splitting of a nucleus into smaller nuclei, accompanied by a release of neutrons and large amount of energy (exothermic)
What elements are used in fission
Commonly used isotopes are Uranium-235 and Plutonium-239
Example of Fission Formula
235/92 U + 1/0 n->92/36 Kr +141/56 Ba +3 1/0 n + Energy
Fission photo
YAy
E=mc^2
Einstein's equation proposing that energy has mass; E is energy, m is mass, and c is the speed of light
In chemical equations, mass change is
nearly undetectable
The mass of the nucleus
less than the sum of the masses of its individual nucleons
Mass Defect
Mass of the constituent (nucleons)-mass of the nucleus
In mass defect, the missing mass is
converted into energy, which is used to hold the nucleus together-tighter, lighter separate, heavier
Fission requires
slow-moving neutrons
Important fissionable nuclei
U-233, U-235, Pu-239
Chain Reaction
One nuclear reaction leads to one or more others
Mass defect is also known as
mass deficiency
How does fission occur
Distance too big; strong force weakens, +/+ repulsion takes over, released n, free to split more nuclei
Control rods are commonly made of
boron or cadmium, and can be lowered into the reactor
Control rods
Slow the reaction by absorbing neutrons, so there is less available to trigger fission, stopping chain rxn
Moderators are usually
water, graphite
Moderators
help slow neutrons down in the reactor so that they can be absorbed by uranium atoms
Critical mass
the mass of fissionable material required to maintain a chain reaction at a constant rate
Supercritical Mass
the mass above which the chain reaction accelerates
Supercritical mass is used in
bombs
Benefits of nuclear energy
1.) No air pollution
2.) Small volume of material consumed
3.) Breeder reactors
Breeder reactors
Reactors that generate new fissionable material at a greater rate than the original fuel is consumed
Example of breeder reactors
U-238 to Pu-239 (Non fissionable transmutated to fissionable.)
Problems with nuclear energy
Nuclear waste (storage and transport)
Fusion
occurs when nuclei combine to produce a nucleus of greater mass
Fusion is known as a
exothermic process (much more energy than fission_
Example of fusion equation
3/1H+2/1H->4/2He+1/0n+Energy
Fusion equations have
smaller isotopes, always hydrogen
Artificial transmutation equations
Any other element besides ones typically used-not get energy
Fusion is also called
thermonuclear reactions
Products of fusion
generally not radioactive
Fusion requires
very high temperatures
Fusion currently
has not reached ignition, meaning, it requires more energy than it produces
Tokamaks
Use magnetic fields to contain and heat the plasma reactives
Dating
Carbon-14 is measured in dead organisms to find out when it was last alive based on its half life
medical
certain radioisotopes are useful because they contain short half lives and are quickly removed from the body
Iodine-131
Used to detect and treat thyroid disorders
Cobalt-60
emits gamma rays and is used to treat cancer-prostrate
Technetium-99
Detects cancerous brain tumors
Fusion formulas have
only hydrogen, produce helium
Fission formula
large creates large, neutrons on products side