Minor and Short-Acting Analgesics, Including Opioid Combination Products
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
Acetaminophen (APAP)
Mechanism of Action, risks and precautions
Acts centrally with weak COX inhibition, lacks peripheral anti-inflammatory effects. Its anti-pyretic activity may be secondary to blockade of prostaglandin (PG) production and inhibition of PG endoperoxide H2 synthase and COX centrally.
Possibly enhances endogenous opioid activity.
Decreases β-endorphin activity and modulates serotoninergic and cannabinoid pathways.
Risks and Precautions:
Hepatotoxicity: Excess dosing saturates safe metabolism pathways → buildup of NAPQI, a toxic metabolite.
Renal toxicity in chronic use.
Increased ALT even at therapeutic doses.
Potential interactions: Alcohol, anticonvulsants (e.g., phenytoin), CYP2E1 inducers.
Codeine MOA, risks/SE and organ system impact
Mechanism:
Weak μ-opioid receptor agonist.
Prodrug: Metabolized to morphine via CYP2D6 (only ~3% conversion).
Also metabolized to codeine-6-glucuronide, which is active.
Risks:
Variable efficacy based on genetics (CYP2D6 polymorphisms).
Side effects: Cognitive impairment, sedation, constipation, euphoria, dizziness.
Organ System Impact:
CNS: Sedation, dizziness.
GI: Constipation.
Respiratory: Suppression possible at higher doses.
Hydrocodone- similar structure to ___but 6-8x more potent
MOA, risks and organ system impact
codeine
Mechanism:
Prodrug metabolized to hydromorphone (active) via CYP2D6, and to noroxycodone (inactive) via CYP3A4.
Risks:
Liver toxicity due to combination with APAP[tylenol].
Physical dependence and tolerance.
Frequently misused and abused.
Organ System Impact:
CNS: Analgesia, sedation, euphoria.
Respiratory: Depression.
GI: Constipation.
Liver: APAP-induced hepatotoxicity if combined.
Oxycodone MOA, risks and organ system impact
Mechanism:
μ-opioid receptor agonist; partial κ-opioid receptor activity.
Metabolized by CYP2D6 (to oxymorphone) and CYP3A4 (to noroxycodone).
Risks:
High abuse potential due to strong CNS effects.
Can be used for opioid rotation.
Organ System Impact:
CNS: Potent analgesia, euphoria.
Respiratory: Significant depression.
GI: Constipation.
Liver: Metabolized hepatically but not as toxic as APAP.
this medications is a Weak opioid that was withdrawn from the market due to cardiac toxicity (accumulation of norpropoxyphene → torsades de pointes).
Propoxyphene
Tramadol
Mechanism:
Weak synthetic ______ receptor agonist.
Inhibits ___&____ reuptake. Activation of these descending pain inhibition pathways stimulates ____that inhibit the transmission of painful stimuli in the dorsal horn by the action of ____!
More recently, noted actions include a local anesthetic effect, anti-inflammatory effects in rat experimental models, and reduction of substance P levels in human synovial fluid
Metabolized to ___, a potent opioid metabolite. 300 times higher affinity for the μ receptor than the parent compound does and is up to ___times more potent in producing analgesia.
μ-opioid [affinity for the mu-receptor is 6000 times weaker than that of morphine]
norepinephrine and serotonin
interneurons
endogenous opioids
M1 [O-desmethyltramadol]
six
risks associated w/ tramadol
Risks:
The most commonly reported side effects include: nausea, vomiting, dizziness, fatigue, sweating, dry mouth, drowsiness, sedation, and orthostatic hypotension
Other more severe side effects include angioedema, bleeding complications because of the increased effect of oral anticoagulants, and serotonin toxicity
Serotonin syndrome risk (especially with SSRIs, MAOIs and SNRIs).
Seizures, especially in predisposed individuals.
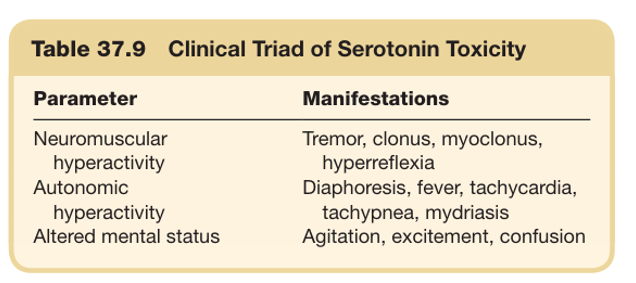
what is the clinical triad of serotonin syndrome ?
Exogenous glucocorticoid administration has been shown to have a suppressive effect on the ______
The use of steroids for the treatment of painful conditions is largely based on the premise that there is an ____role in the mediation of pain
Steroids suppress or prevent cell-mediated ___responses and decrease or prevent ___response to the inflammatory process.
The most common side effects, with approximate prevalence, include:
hypothalamic-pituitary-adrenal (HPA) axis
inflammatory
immune ; tissue
weight gain (70%), skin bruising (53%), sleep disturbance (45%), mood symptoms (42%), cataracts (15%), acne (15%), and fractures (12%).
Caffeine: CNS stimulant; enhances analgesia.
Caffeine exhibits antinociceptive effects and analgesic properties when used in combination with ____
Caffeine (65 to 130 mg) was shown to increase the potency of other analgesics by ___
Centrally, caffeine may increase ___&___, as well as act as a vasoconstrictor of vessels, thereby contributing to its analgesic effect in certain ______ conditions.
opioid analgesics
40%
dopamine and norepinephrine; headache
T/F: topical preparations have considerably higher potential for systemic adverse effects and organ toxicity
FALSE
topical preparations have considerably less potential for systemic adverse effects and organ toxicity
Transdermal methods deliver medication through percutaneous absorption, with the goal of achieving similar therapeutic systemic levels as ______.
Transdermal pharmacotherapies can be administered ____to the site of injury
active oral preparations do
distal
Topical agents use _____delivery to target the site of application specifically.
The sites of action of topical agents are the
________ underlying the site of application
cutaneous
soft tissues and peripheral nerves
different proposed peripheral mechanisms of topical NSAID analgesic activity
inhibition of PGE synthesis, the lipoxygenase pathway, and excitatory amino acids, as well as modulation of G protein–mediated signal transduction
Commonly used topical agents for neuropathic pain :
are amitriptyline, ketamine, lidocaine, and capsaicin
MOA of capsaicin
The spicy ingredient in chili peppers has been used to relieve neuropathic pain, uremic pruritus, and bladder overactivity, as well as to provide analgesia.
An initial excitatory phase (pain and inflammation), mediated by activation of the TRPV1 receptor, is followed by a secondary analgesic phase that has been attributed to long-term desensitization of nociceptors and depletion of substance P
Risk factors for opioid addiction
Genetics (e.g., CYP2D6 variants)
age 16-45
History of substance abuse [personal or family]
Psychiatric comorbidities
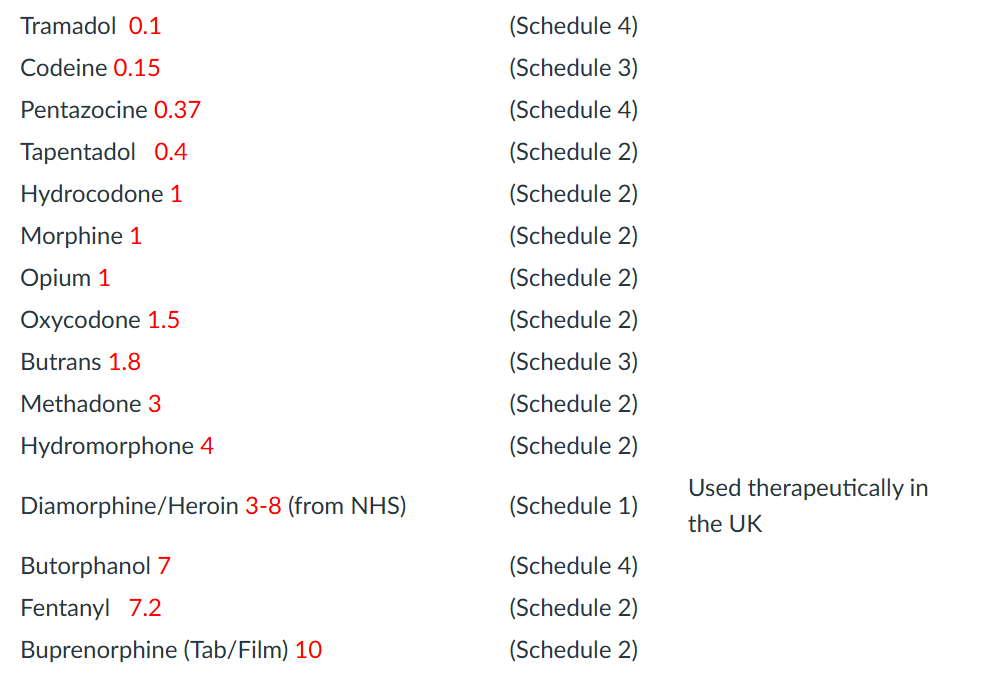
morphine Equivalents doses w/ common meds