Orgo CH11 Practice Problems
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
33 Terms
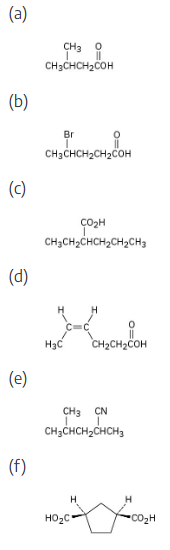
Give IUPAC names for the following compounds:
Carboxylic acids are named by replacing –e of the corresponding alkane with –oic acid.
The carboxylic acid carbon is C1.
When –CO2H is a substituent of a ring, the suffix –carboxylic acid is used; the carboxyl carbon is not numbered in this system.
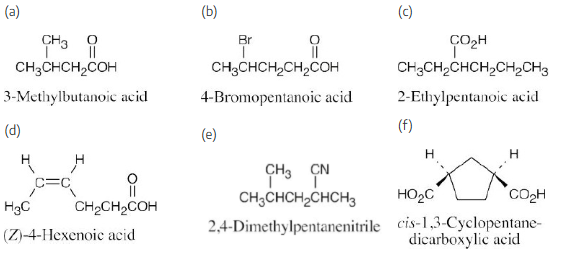
Draw structures corresponding to the following IUPAC names:
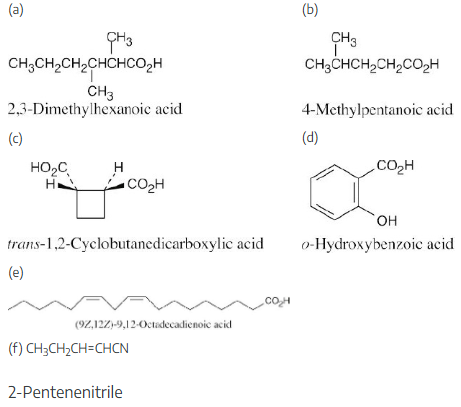
(a) 2,3-Dimethylhexanoic acid
(b) 4-Methylpentanoic acid
(c) trans-1,2-Cyclobutanedicarboxylic acid
(d) o-Hydroxybenzoic acid
(e) (9Z,12Z)-9,12-Octadecadienoic acid
(f) 2-Pentenenitrile
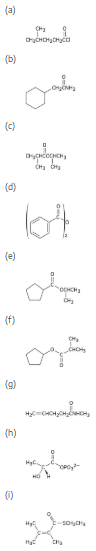
Give IUPAC names for the following substances:
Table 11.1 lists the suffixes for naming carboxylic acid derivatives. The suffixes used when the functional group is part of a ring are in parentheses.
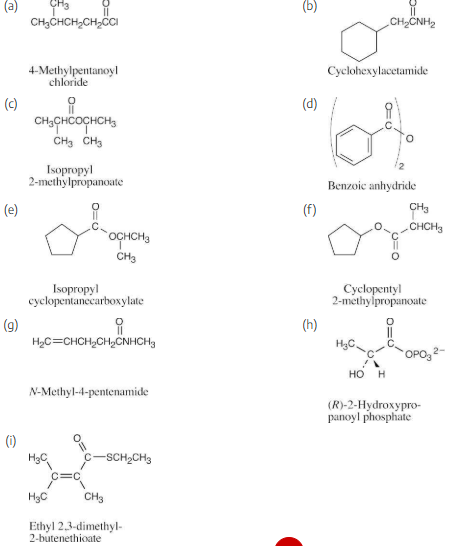
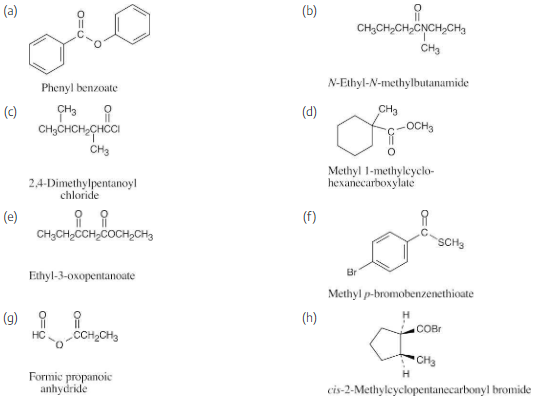
Draw structures corresponding to the following names:
(a) Phenyl benzoate
(b) N-Ethyl-N-methylbutanamide
(c) 2,4-Dimethylpentanoyl chloride
(d)Methyl 1-methylcyclohexanecarboxylate
(e) Ethyl 3-oxopentanoate
(f) Methyl p-bromobenzenethioate
(g) Formic propanoic anhydride
(h) cis-2-Methylcyclopentanecarbonyl bromide
Assume you have a mixture of naphthalene and benzoic acid that you want to separate. How might you take advantage of the acidity of one component in the mixture to effect a separation?
Naphthalene is insoluble in water and benzoic acid is only slightly soluble. The salt of benzoic acid is very soluble in water, however, and we can take advantage of this solubility in separating naphthalene from benzoic acid.
Dissolve the mixture in an organic solvent, and extract with a dilute aqueous solution of sodium hydroxide or sodium bicarbonate, which will neutralize benzoic acid.
Naphthalene remains in the organic layer, and all the benzoic acid, now converted to the benzoate salt, is in the aqueous layer. To recover benzoic acid, remove the aqueous layer, acidify it with dilute mineral acid, and extract with an organic solvent.
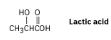
Which would you expect to be a stronger acid, the lactic acid found in tired muscles or acetic acid? Explain.
You would expect lactic acid to be a stronger acid because the electron-withdrawing inductive effect of the hydroxyl group can stabilize the lactate anion.
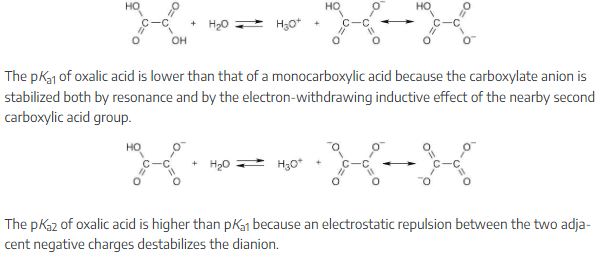
Dicarboxylic acids have two dissociation constants, one for the initial dissociation into a monoanion and one for the second dissociation into a dianion. For oxalic acid, HO2C–CO2H, the first ionization constant is pKa1 = 1.2 and the second ionization constant is pKa2 = 4.2.
Why is the second carboxyl group far less acidic than the first?
The pKa1 of oxalic acid is lower than that of a monocarboxylic acid because the carboxylate anion is stabilized both by resonance and by the electron-withdrawing inductive effect of the nearby second carboxylic acid group.
The pKa2 of oxalic acid is higher than pKa1 because an electrostatic repulsion between the two adjacent negative charges destabilizes the dianion.
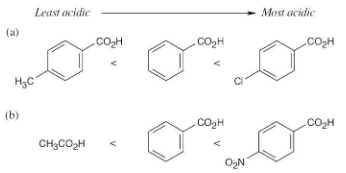
Rank the following compounds in order of increasing acidity. Don’t look at a table of pKa data to help with your answer.
(a) Benzoic acid, p-methylbenzoic acid, p-chlorobenzoic acid
(b) p-Nitrobenzoic acid, acetic acid, benzoic acid
Remember that electron-withdrawing groups increase carboxylic acid acidity, and electron-donating groups decrease carboxylic acid acidity. Benzoic acid is more acidic than acetic acid.
How would you prepare CH3CH2CH2CO2H from CH3CH2CH2Br?
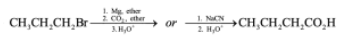
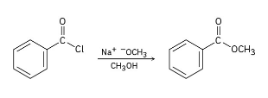
Show the mechanism of the following nucleophilic acyl substitution reaction, using curved arrows to indicate the electron flow in each step:
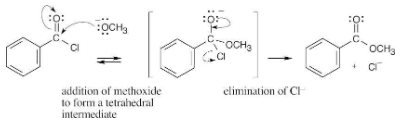
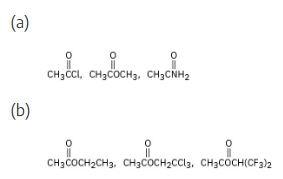
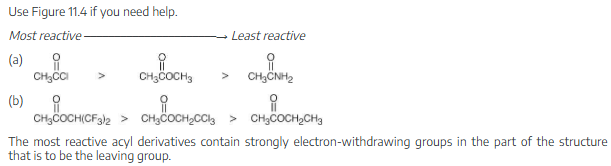
Rank the compounds in each of the following sets in order of their expected reactivity toward nucleophilic acyl substitution:
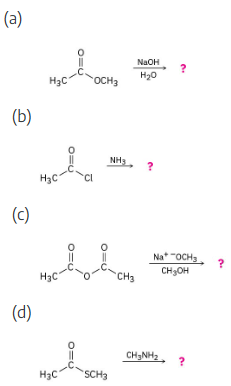
Predict the products of the following nucleophilic acyl substitution reactions:

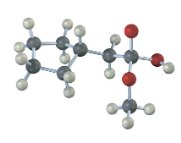
The following structure represents a tetrahedral alkoxide ion intermediate formed by addition of a nucleophile to a carboxylic acid derivative. Identify the nucleophile, the leaving group, the starting acid derivative, and the ultimate product.
The structure represents the tetrahedral intermediate in the reaction of methyl cyclopentylacetate with hydroxide, a nucleophile. The products are cyclopentylacetate anion and methanol.
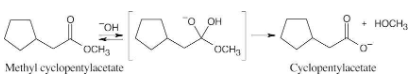
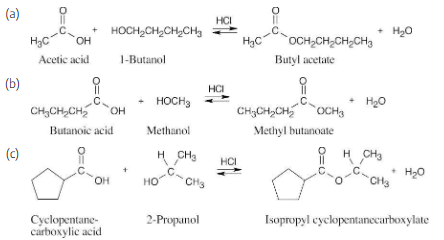
How might you prepare the following esters from the corresponding acids?
In Fischer esterification, an alcohol undergoes a nucleophilic acyl substitution with a carboxylic acid to yield an ester. The mineral acid catalyst makes the carboxyl group of the acid more electrophilic. Predicting the products is easier if the two reagents are positioned so that the reacting functional groups point towards each other.
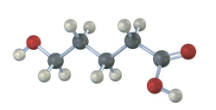
If the following molecule is treated with acid catalyst, an intramolecular esterification reaction occurs. What is the structure of the product? (Intramolecular means within the same molecule.)
Under Fischer esterification conditions, many hydroxycarboxylic acids can form intramolecular esters (lactones)
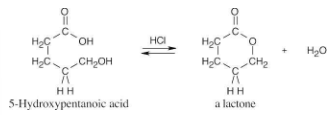
How might you prepare 2-phenylethanol from benzyl bromide? More than one step is needed.
The alcohol product can be formed by reduction of a carboxylic acid with LiAlH4. The carboxylic acid can be synthesized either by Grignard carboxylation or by nitrile hydrolysis. The product can also be formed by a Grignard reaction between benzyl bromide and formaldehyde.
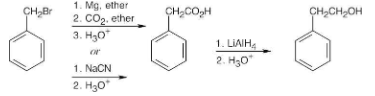
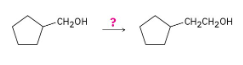
How might you carry out the following transformation? More than one step is needed.
After treating the initial alcohol with PBr3, the same steps as used in the previous problem can be followed. A Grignard reaction between the cycloalkylmagnesium bromide and formaldehyde also yields the desired product.
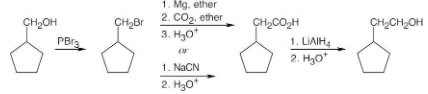
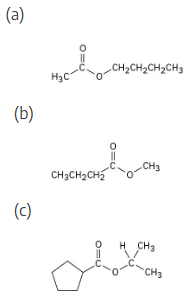
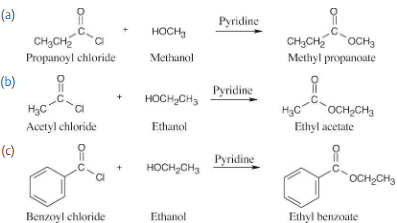
How might you prepare the following esters using a nucleophilic acyl substitution reaction of an acid chloride?
(a) CH3CH2CO2CH3
(b) CH3CO2CH2CH3
(c) Ethyl benzoate
Pyridine neutralizes the HCl byproduct by forming pyridinium chloride. This neutralization removes from the product mixture acid that might cause side reactions. As mentioned previously, positioning the reacting groups so that they face each other makes it easier to predict the products.
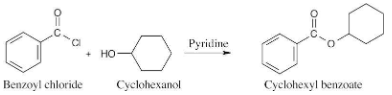
Which method would you choose if you wanted to prepare cyclohexyl benzoate—Fischer esterification or reaction of an acid chloride with an alcohol? Explain.
As explained in the text, only simple, low boiling alcohols are convenient to use in the Fischer esterification reaction. Thus, reaction of cyclohexanol with benzoyl chloride is the preferred method for preparing cyclohexyl benzoate.
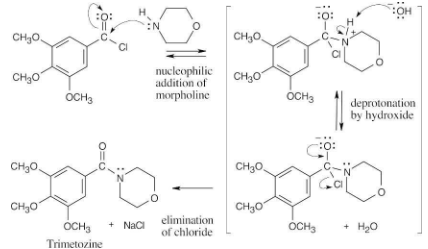
Write the mechanism of the reaction just shown between 3,4,5-trimethoxybenzoyl chloride and morpholine to form trimetozine. Use curved arrows to show the electron flow in each step.
How could you prepare the following amides using an acid chloride and an amine or ammonia?
(a) CH3CH2CONHCH3
(b)N,N-Diethylbenzamide
(c) Propanamide
An extra equivalent of base must be added to neutralize the acid produced in these reactions. In (a) and (b), two equivalents of the amine may be used in place of NaOH.
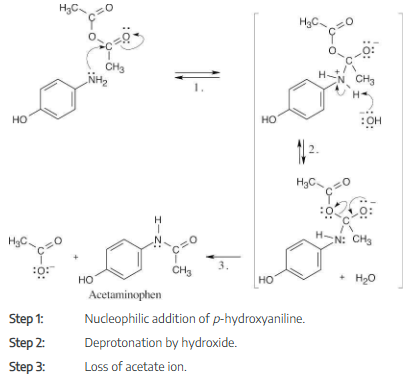
Write the mechanism of the reaction between p-hydroxyaniline and acetic anhydride to prepare acetaminophen.
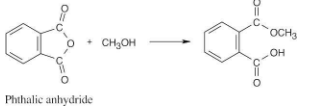
What product would you expect from reaction of one equivalent of methanol with a cyclic anhydride, such as phthalic anhdride (1,2-benzenedicarboxylic anhydride)? What is the fate of the second “half” of the anhydride?
The second half of the anhydride becomes a carboxylic acid.
Why is the saponification of an ester irreversible? In other words, why doesn’t treatment of a carboxylic acid with an alkoxide ion yield an ester?
Acidic hydrolysis of an ester is a reversible reaction because the products are an alcohol and a carboxylic acid. Basic hydrolysis of an ester is irreversible because its products are an alcohol and a carboxylate anion, which has a negative charge and does not react with nucleophiles.

What product would you expect from the reaction of butyrolactone with LiAlH4?
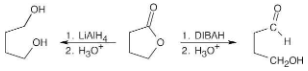
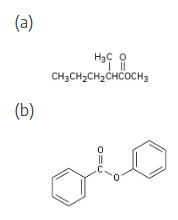
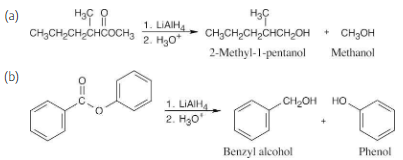
Show the products you would obtain by reduction of the following esters with LiAlH4:
Lithium aluminum hydride reduces an ester to form two alcohols.
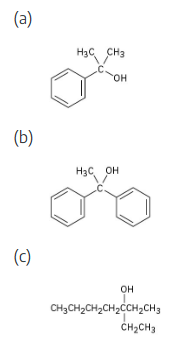
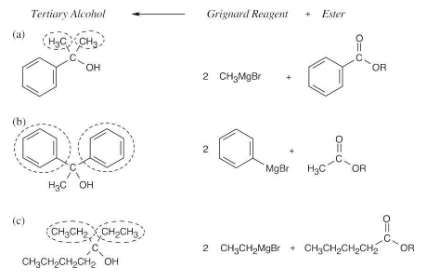
What ester and what Grignard reagent might you start with to prepare the following alcohols?
Remember that Grignard reagents can only be used with esters to form a tertiary alcohol that has two identical substituents. Identify these two substituents, which come from the Grignard reagent, and work backward to select the ester (the alkyl group of the ester is unimportant).
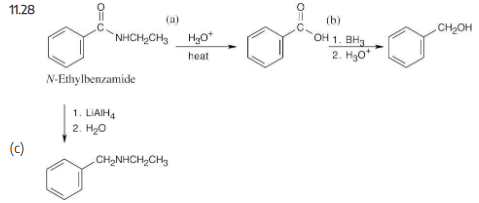
How would you convert N-ethylbenzamide to each of the following products?
(a) Benzoic acid
(b) Benzyl alcohol
(c) C6H5CH2NHCH2CH3
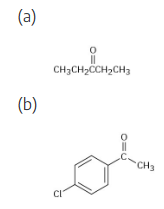
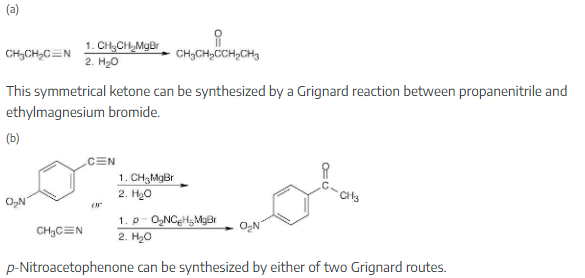
How would you prepare the following carbonyl compounds from a nitrile?
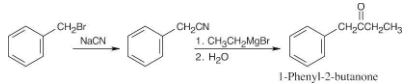
How would you prepare 1-phenyl-2-butanone, C6H5CH2COCH2CH3, from benzyl bromide, C6H5CH2Br? More than one step is needed.
Once you realize that the product results from a Grignard reaction with a nitrile, this synthesis is easy.
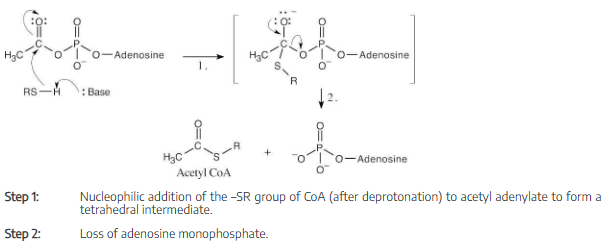
Write the mechanism of the reaction shown in Figure 11.13 between coenzyme A and acetyl adenylate to give acetyl CoA.
Even though the entire molecule of coenzyme A is biologically important, we are concerned in this problem only with the –SH group. The remainder of the structure is represented here as “R”.
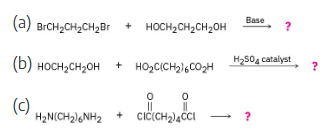
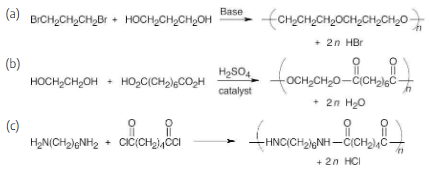
Draw structures of the step-growth polymers you would expect to obtain from the following reactions:
In each example, if n molecules of one component react with n molecules of the other component, a polymer with n repeating units is formed, and 2n small molecules are formed as byproducts; these are shown in each reaction.
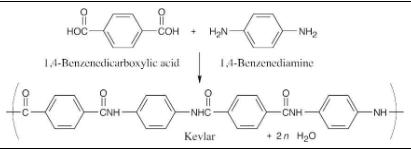
Kevlar, a nylon polymer prepared by reaction of 1,4-benzenedicarboxylic acid (terephthalic acid) with 1,4-benzenediamine (p-phenylenediamine), is so strong that it’s used to make bulletproof vests. Draw the structure of a segment of Kevlar.