Chemistry - Ketone and Aldehydes
1/16
Earn XP
Description and Tags
idk what the hell is happening im deadass cooked.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
Jones Oxidation (Na₂Cr₂O₇)
Strong oxidation of aldehydes → carboxylic acids.
Strong oxidation of 1° alcohols → carboxylic acids.
2° alcohols → ketones.
Cannot stop at aldehyde; pushes fully to acid.
Acetone is solvent.
NaBH₄ Reduction (NaBH₄, EtOH)
Reduces aldehydes & ketones → alcohols.
Does not reduce esters, amides, or carboxylic acids.
Safe with protic solvents (EtOH, MeOH).
Selective for carbonyls in complex molecules.
KMnO4 Oxidation (KMnO₄, heat)
Strong oxidation: aldehydes/1° alcohols → acids.
2° alcohols → ketones.
Cleaves alkenes → carbonyls or acids.
Very aggressive, over-oxidation common.
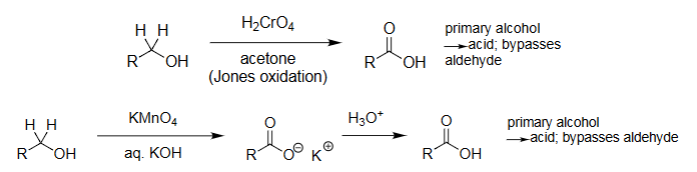
PCC Oxidation (PCC with solvent CH₂Cl₂)
1° alcohols → aldehydes (no over-oxidation).
2° alcohols → ketones.
Works under anhydrous conditions.
Milder than Jones/KMnO₄.

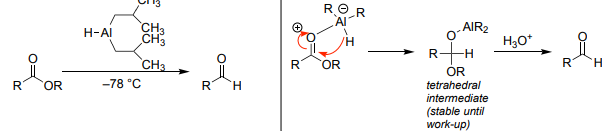
Swern Oxidation (DMSO, (COCl)₂, Et₃N)
1° alcohols → aldehydes.
2° alcohols → ketones.
No heavy metals.
Cold (–78°C) required.

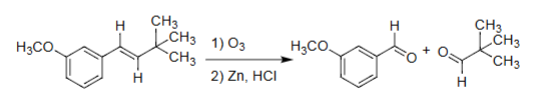
Ozonolysis (O₃ → Zn/HCl or DMS)
Cleaves alkenes → two carbonyls.
Reductive workup → aldehydes/ketones.
Oxidative workup → acids/ketones.
Exact cleavage of double bond.
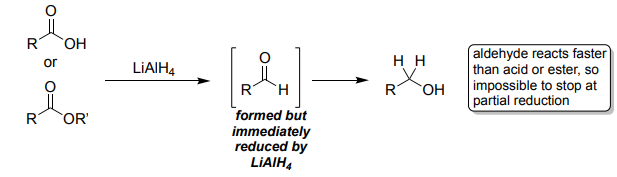
LiAlH₄ Reduction (1. LiAlH₄ 2. H₃O⁺)
Reduces aldehydes → 1° alcohols.
Reduces ketones → 2° alcohols.
Reduces esters/acids/amides → alcohols/amines.
Must be quenched carefully with water after reaction.
Grignard (1. R–MgBr 2. H₃O⁺)
Nucleophilic R⁻ attack on carbonyl carbon.
Aldehydes → 2° alcohols (add one R).
Ketones → 3° alcohols (add one R).
Adds C–C bonds (key carbon chain-building reaction).
Tollens Oxidation (Ag₂O, OH⁻ → H₃O⁺)
Selective aldehyde oxidation → carboxylate → acid.
Leaves alcohols/ketones unchanged.
Produces silver mirror.
Mild & chemoselective.
Wittig Reaction (Ph₃P=CH₂)
Converts carbonyl C=O → C=C.
Aldehydes → terminal alkenes with =CH₂.
Replaces oxygen entirely.
Good for stereoselective alkene formation; opposite of ozonolysis.
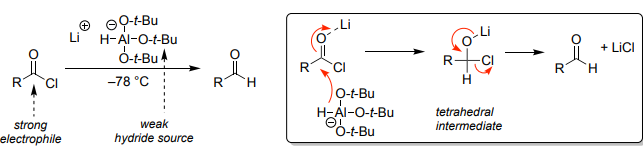
Weak Hydride Reduction (LTBA) (Li(t-BuO)₃, cold)
Selective reduction of acid chlorides → aldehydes.
Stops before alcohol stage.
Requires cold conditions (–78°C).
More selective than LiAlH₄.
DIBAL-H Reduction (DIBAL-H → H₃O⁺)
Esters → aldehydes (controlled low temp).
Excess/warm → alcohols.
Nitriles → aldehydes (via imine).
Temperature-sensitive.
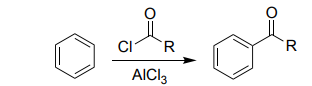
Friedel–Crafts Acylation (RCOCl, AlCl₃)
Benzene → aryl ketone.
No rearrangements.
Product deactivates the ring.
Clean one-substitution reaction.
Alkyne Hydration (HgSO₄, H₂SO₄, H₂O)
Terminal alkyne → methyl ketone (Markovnikov).
Proceeds via enol → keto.
Needs Hg²⁺ catalyst.
Internal alkynes → ketones.
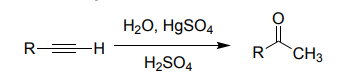
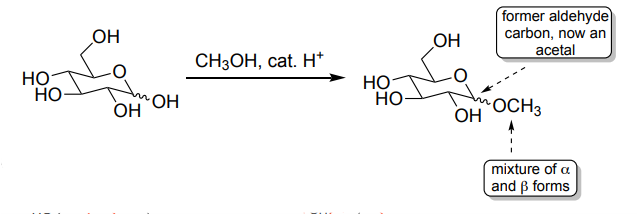
General Acetal Formation (2 ROH, H⁺)
Carbonyl → acetal (protected form).
Hemiacetal intermediate.
Stable in base.
Deprotected with aqueous acid.
Cyclic Acetal Formation (with diol) (HOCH₂CH₂OH, cat. H⁺)
Carbonyl + diol → 5-membered cyclic acetal.
Excellent protecting group.
Stable in base, removable in acid.
Driven by intramolecular ring closure.
Hydrazone Formation (PhNHNH₂, pH 4–5)
Carbonyl → C=N–NHPh (hydrazone).
Dehydration product from hydrazine/hydrazide reacting with an aldehyde or ketone.
Formed via condensation (loss of H₂O).
Product = hydrazone: Carbonyl oxygen replaced by =N–NHPh (or =N–NH₂ for hydrazine).