XII - Carbohydrates and glycoproteins
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Carbohydrates
Carbon based molecules rich in hydroxyl groups, w/ names ending in “ose” Cn(H2O)n
→ component of nucleic acids/DNA/RNA, used as energy source & storage, structural component (cellulose), exoskeleton of insects, cell recognition
Monosaccharides
The simplest form of carbohydrates. Single sugar molecules that are aldehydes or ketones with two or more hydroxyl groups. They are categorized by the number of carbon atoms (e.g., triose C3, pentose C5, hexose C6) and by their functional group (aldose or ketose).
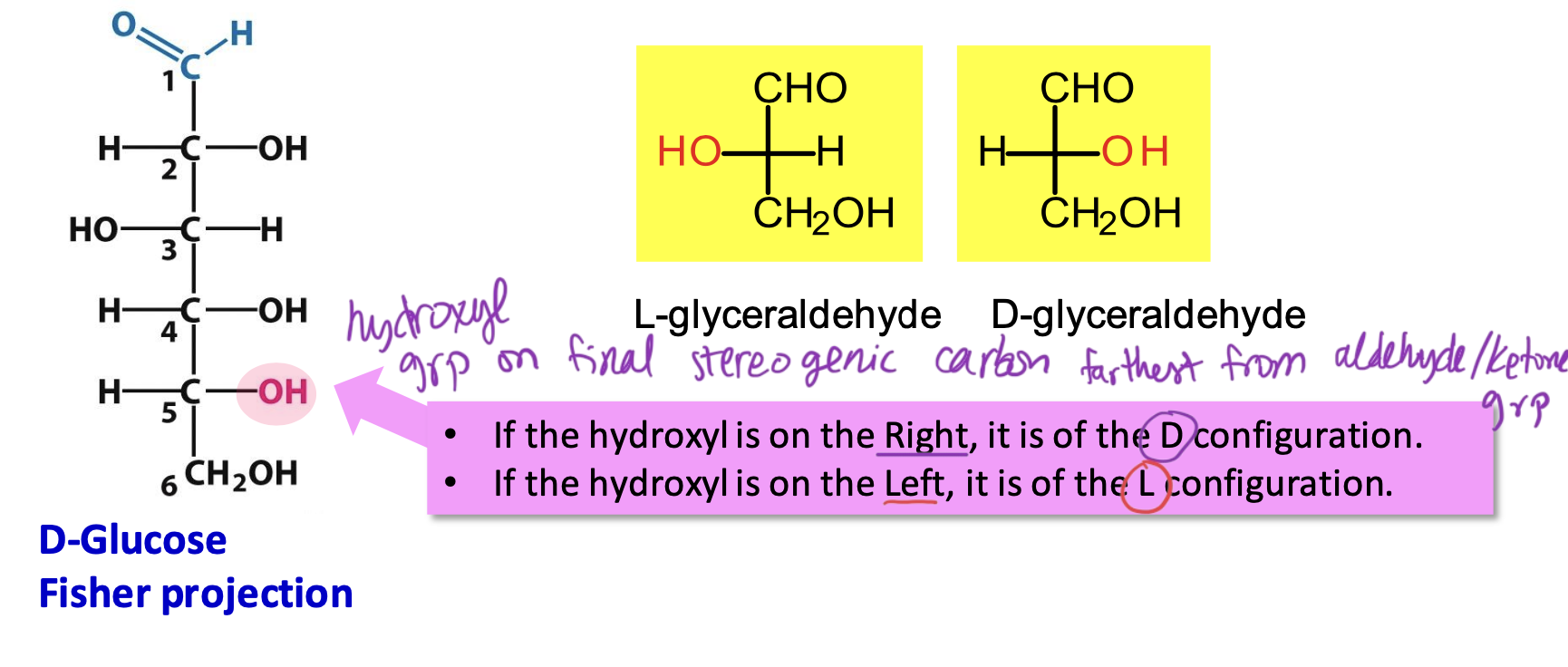
D and L configurations
Based on the hydroxyl group on the final chiral center farthest from aldehyde/ketone group.
→ if OH is on the right, D configuration (most monosaccharides in vertebrates)
→ if OH is on the left, L configuration
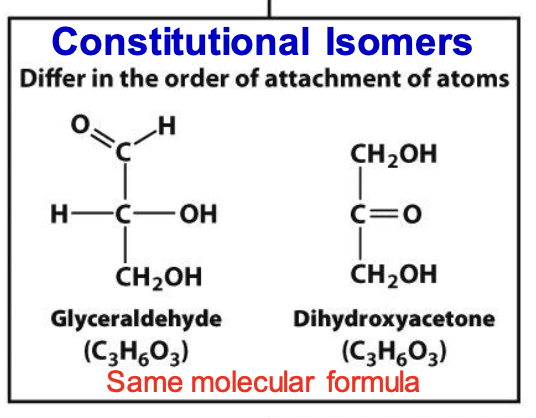
Constitutional isomers
Molecules with the same molecular formula but different connectivity of atoms
Stereoisomers
Molecules connected in the same order that differ in spatial arrangement. In sugars, this includes configurations like D- and L- forms based on chiral centers.
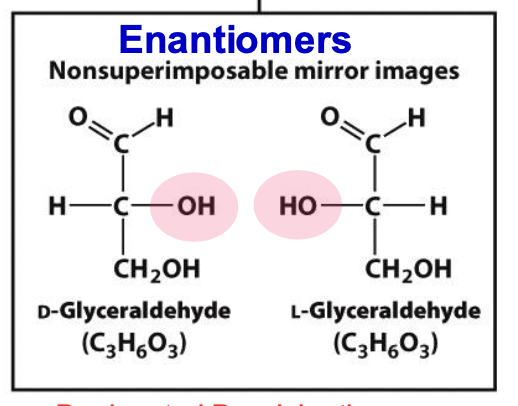
Enantiomers
Stereoisomers that are non superimposable mirror images of each other
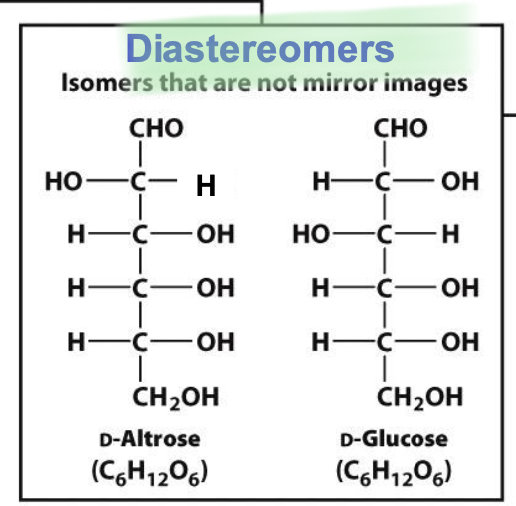
Diastereomers
Stereoisomers that aren’t mirror images of each other
→ D-glucose and D-galactose and differ in configuration @ more than 1 chiral center
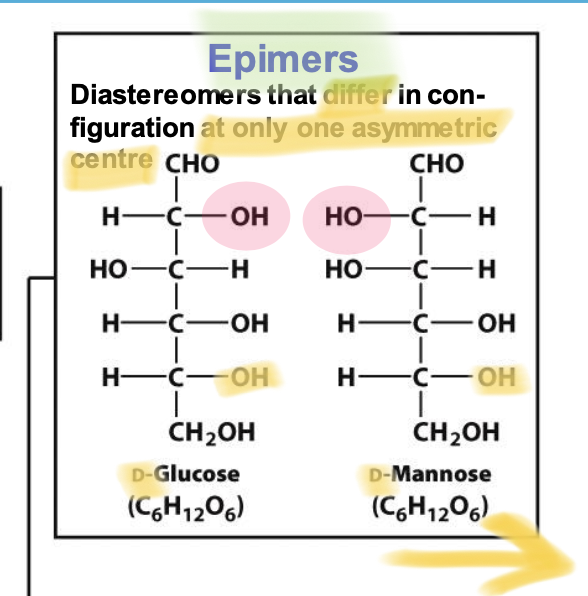
Epimers
Diastereomers that differ in configuration at only one specific chiral carbon. → D-glucose and D-mannose are epimers differing at the C2 carbon.
Anomers
A subtype of epimers found in cyclic sugars, differing in configuration at the anomeric carbon (C1 for aldoses or C2 for ketoses). The two forms are designated as α and β.
Forms of D-glucose
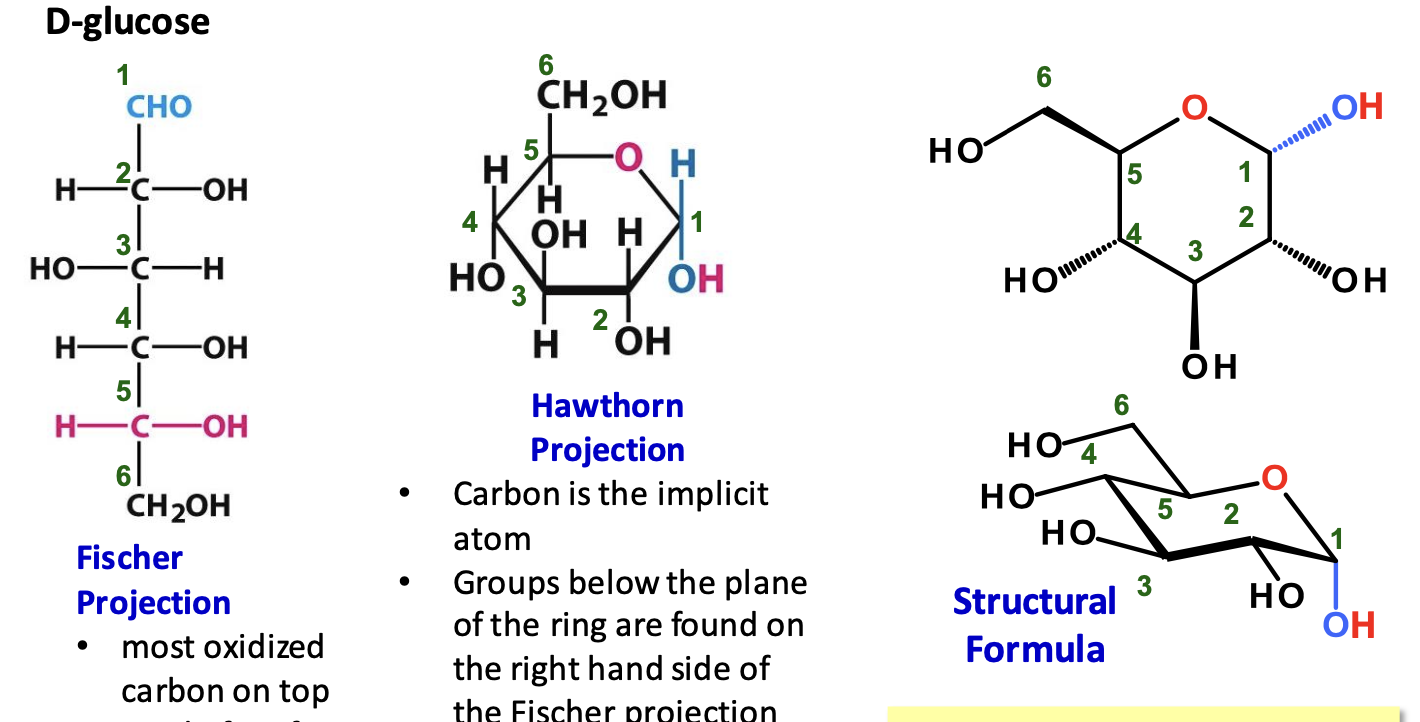
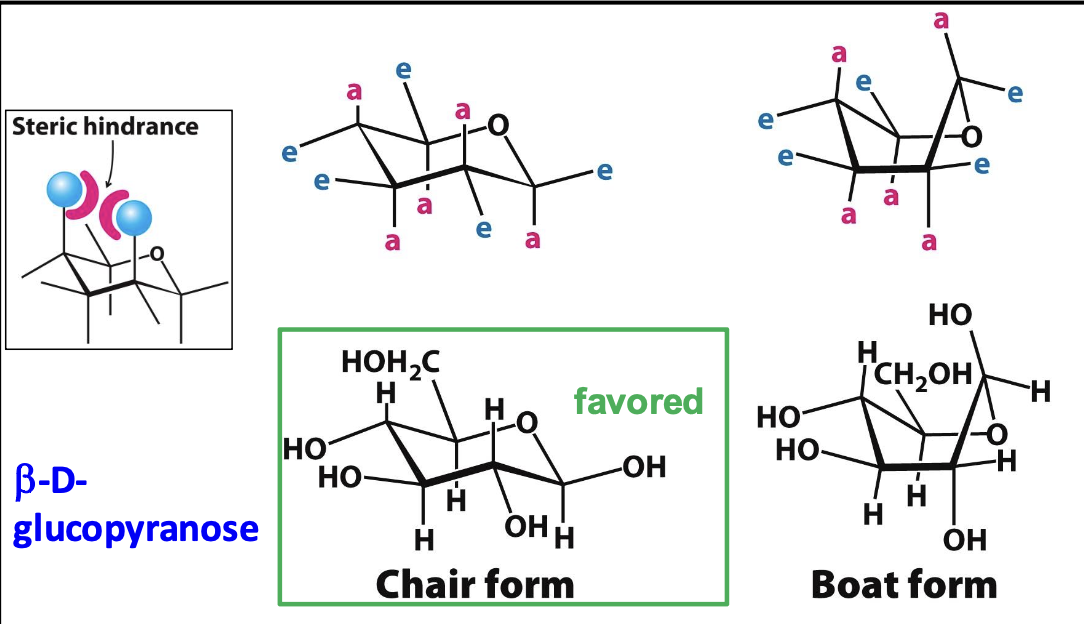
β-D-Glucose Stability
β-D-glucose is the most stable sugar due to its ability to adopt a chair conformation where bulky groups are equatorial, minimizing steric hindrance & diaxial interactions
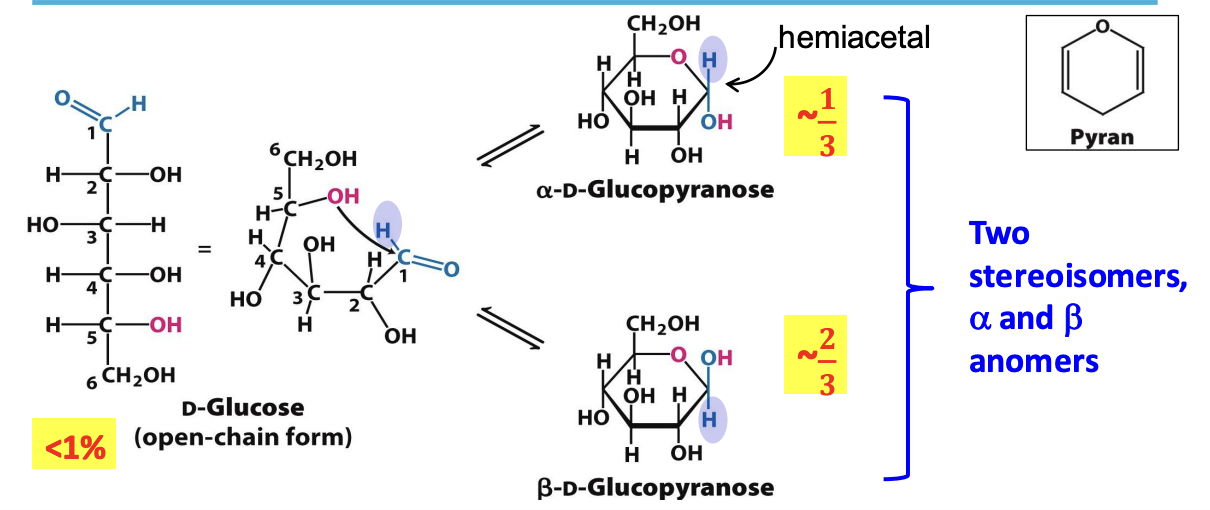
Hemiacetal
Formed by a rx between aldehyde @ C1 and OH @ C5 which creates a new pair of diastereomers w/ a new chiral center at the anomeric carbon
✦ α form has OH @ C1 on opposite of C6 (∼33%)
✦ 𝛽 form has OH @ C1 on the same side as C6 (∼66%)
✧ mutarotation: interconversion of 𝛼 and 𝛽 anomers
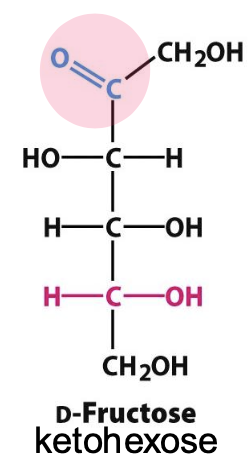
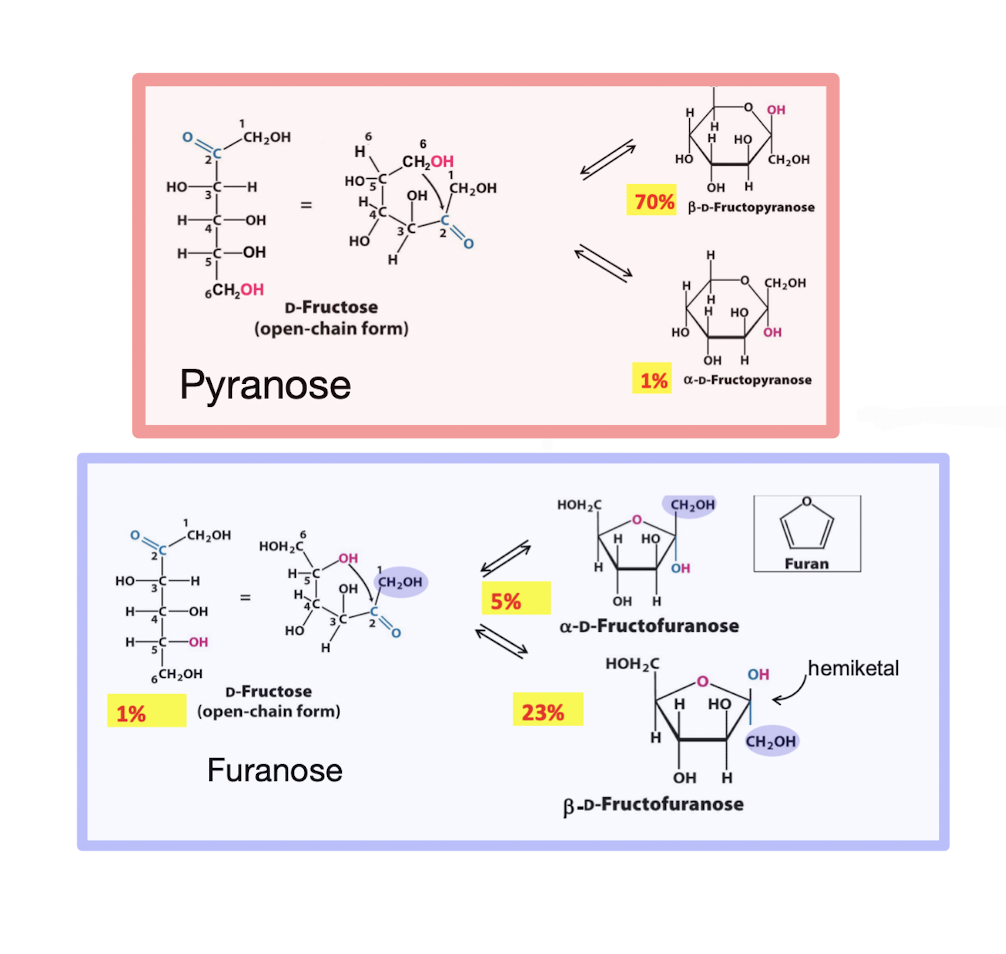
Hemiketal
Formed by a rx between a ketone @ C2 and OH @ C5, forming a pair of anomers that differ only in stereochemistry around hemiketal carbon. Forms 5-membered furanose rings, but can form 6-membered pyranose rings when less sterically hindered
✦ 𝛼 form has OH opposite C6
✦ 𝛽 form has OH on the same side as C6
✧ 𝛽-fructofuranose reduces steric hindrance, but 𝛽-fructopyranose is much more stable in solution (favorable chair conf.)
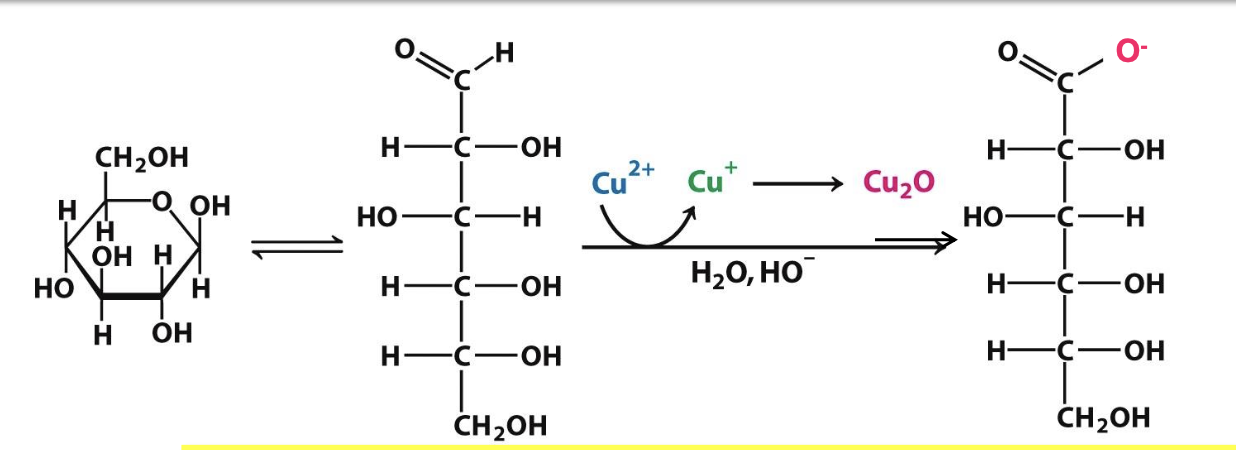
Reducing sugar
A sugar capable of acting as a reducing agent due to a free aldehyde/ketone → can react w/ oxidizing agents
All monosaccharides are reducing sugars, while only some disaccharides are, depending on the glycosidic linkage (able to adopt a linear structure in solution)
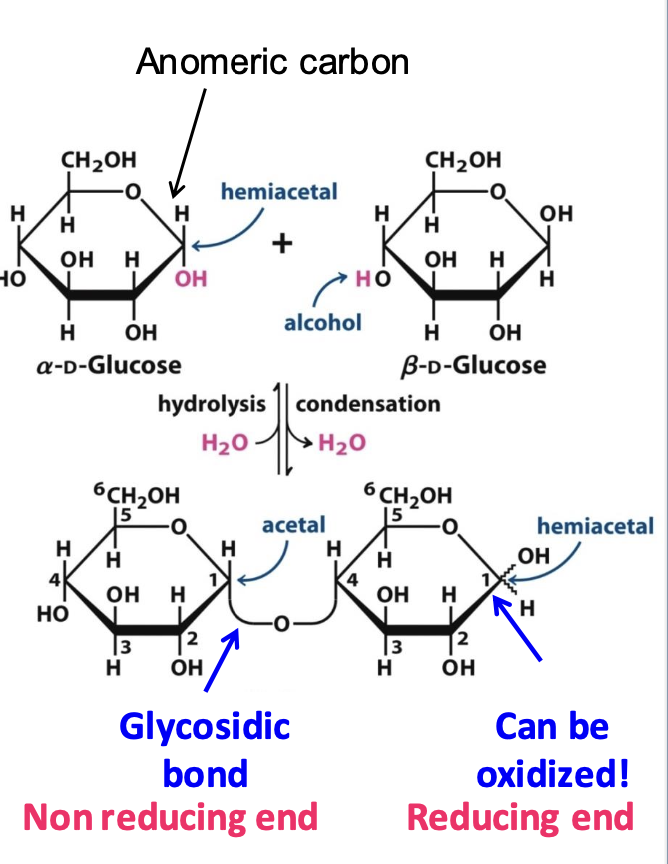
Glycosidic bond
Covalent bond that links monosaccharides together. It forms when a OH group of one is attached to an anomeric carbon (hemiacetal) of another. This bond can be reversed by hydrolysis.
✦ α-Glycosidic Bond: The OH group on the anomeric carbon is trans to the CH₂OH group.
✦ β-Glycosidic Bond: The hydroxyl group on the anomeric carbon is cis to the CH₂OH group.
Naming disaccharides
Start with the non-reducing sugar on the left, specify α or β at the anomeric carbon, indicate D- or L-configuration, and describe the linkage. Then, name the second residue.
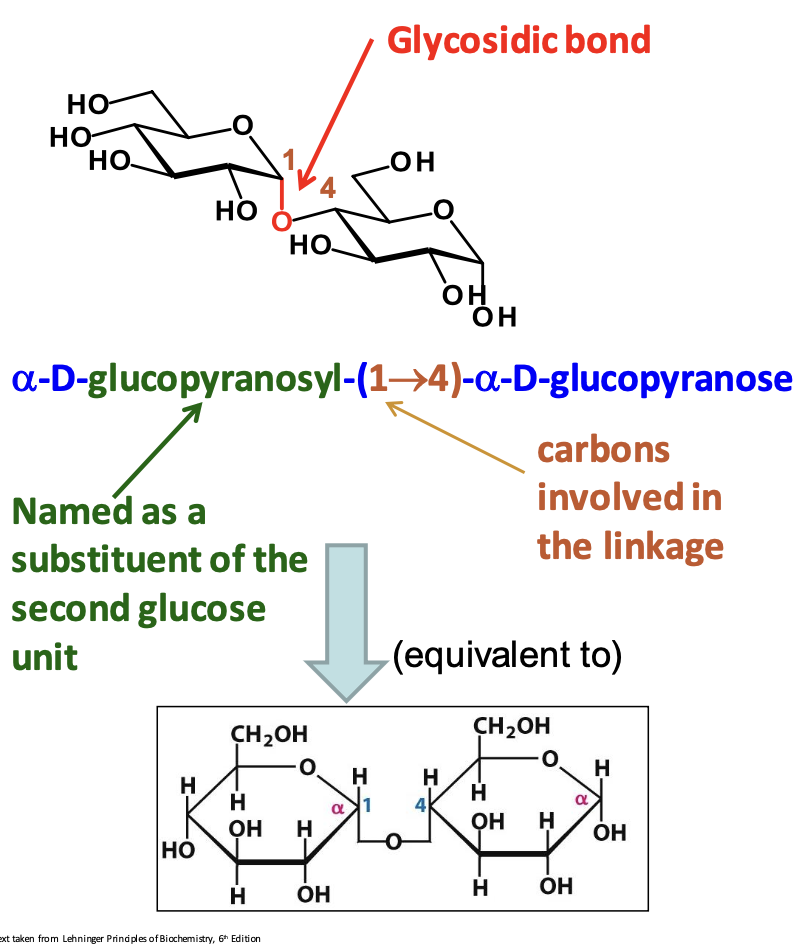
Why does glucose have to be stored as a polysaccharide?
Having a large concentration of glucose in the cell would cause water to diffuse into the cell by osmosis = osmotic imbalance, which would cause the cell to burst
Differences Between Maltose and Lactose
Maltose and lactose are disaccharides that differ in their monosaccharide composition and glycosidic linkages.
✦ Maltose - formed from two glucose molecules (α-1,4 linkage)
✧ Lactose - consists of galactose and glucose (β-1,4 linkage).
Glycogen vs. Cellulose
Glycogen is a branched glucose polymer with α-1,4 and α-1,6 linkages used for energy storage in animals
Cellulose is an unbranched β-1,4 linked glucose polymer that provides structural support in plants.
Storage of sugars in animals and plants
Animals: stored as glycogen, found in liver & muscles, contains 𝛼(1→4) and 𝛼(1→6) every ∼12 glucose units
Plants: stored as 2 homopolymers of starch, which is composed of amylose (𝛼 (1→4) and linear) and amylopectin (similar to glycogen but w/ less branching)
Glycoproteins
Proteins with covalently attached carbohydrate chains, classified by the ratio of protein to sugar. They play roles in cell recognition, adhesion, and immune responses. Types include:
Glycoproteins: Mostly protein by weight, often on cell surfaces for adhesion
Proteoglycans: Mostly carbohydrate, structural components or lubricants. Attached to GAGs (glycoaminoglycans)
Mucoproteins (Mucins): Predominantly carbohydrate, key components in mucus. Attached to carb by N-acetylgalactosamine
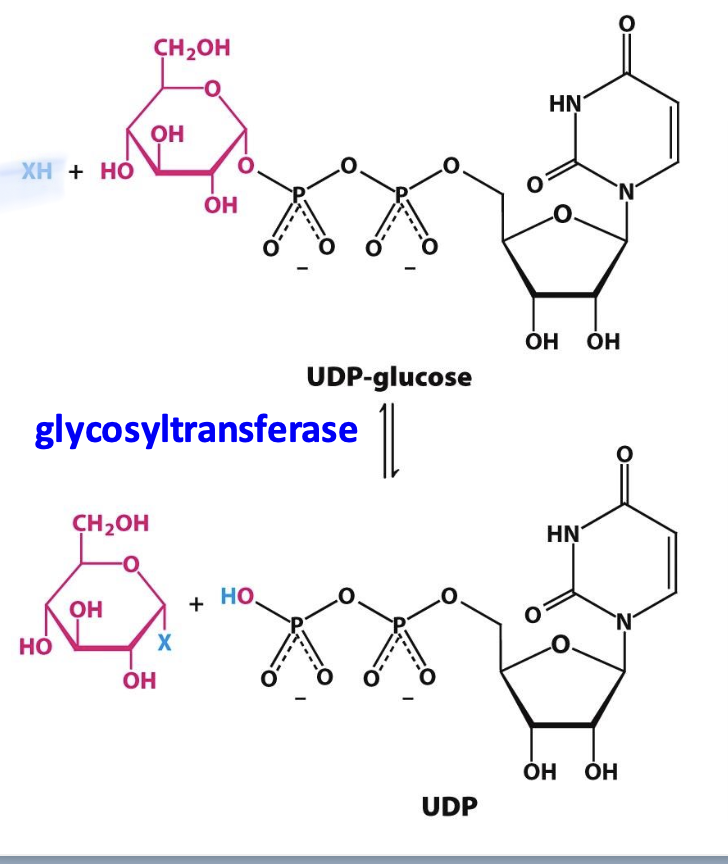
Glycosyltransferases
Enzymes that catalyze the formation of glycosidic bonds in polysaccharides. Monosaccharide substrates are first activated by attachment to UDP (uridine diphosphate)
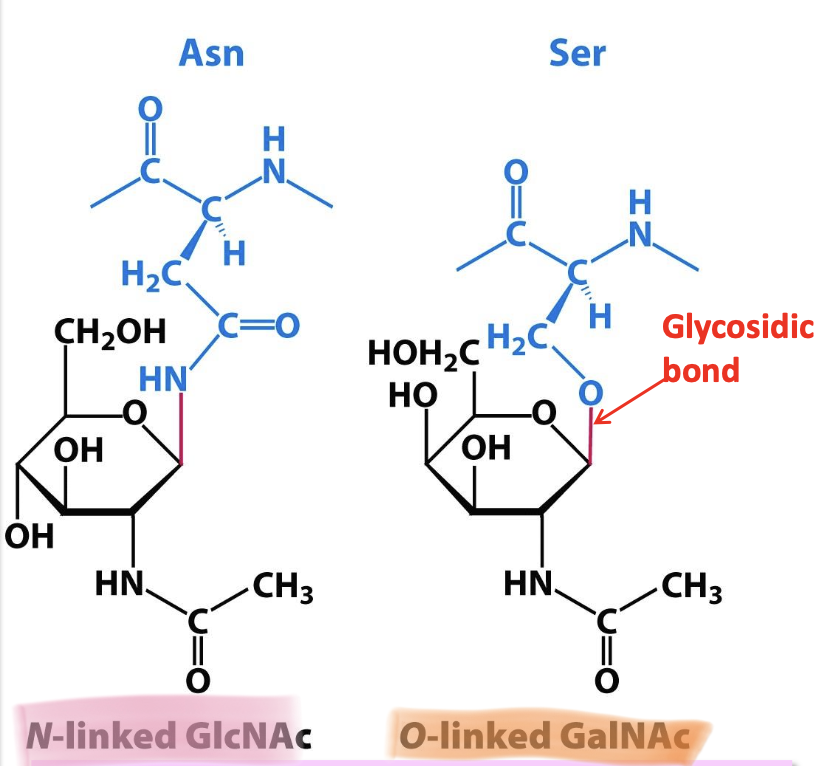
N and O-linked glycoproteins
✦ N-linked: carbohydrate is attached to N atom in the Asn side chain. This Asn must be apart of the consensus sequence Asn-X-Ser/Thr (X ≠ P). Occurs in the ER + Golgi.
✦ O-linked: carbohydrate is attached to O atom in Ser/Thr side chain. Occurs only in the Golgi.
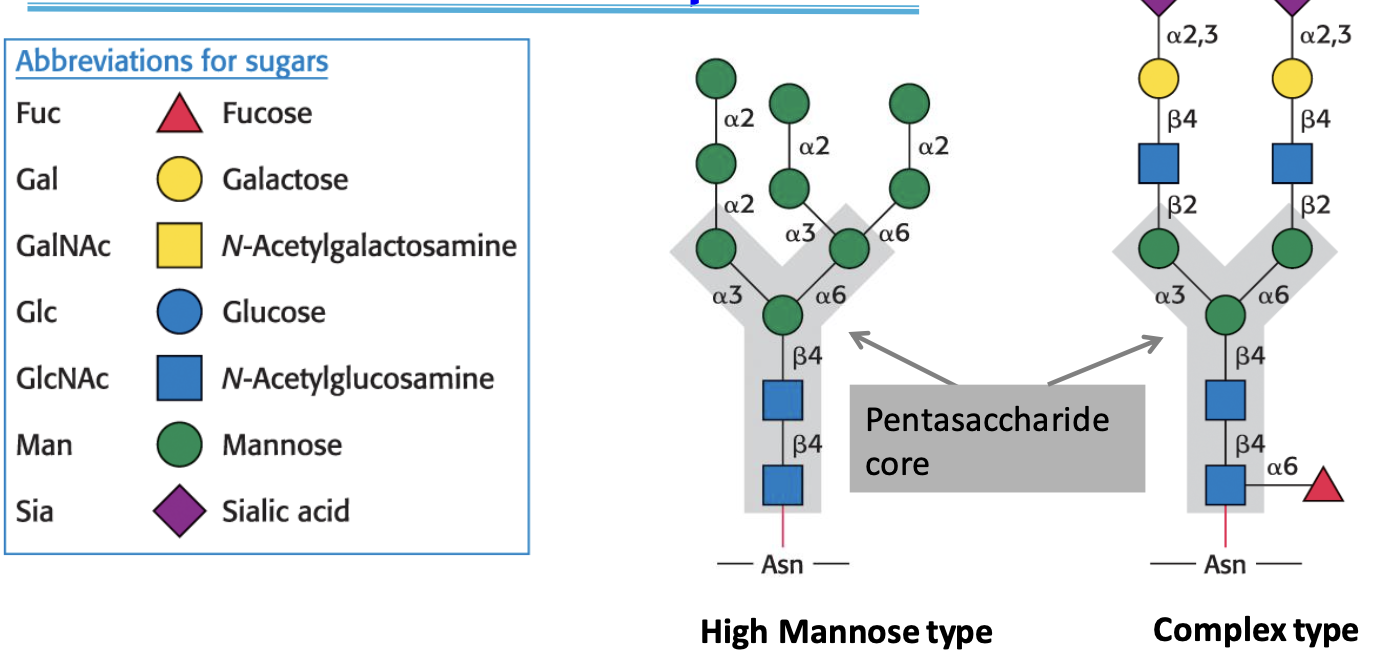
N-Glycans
All N-linked oligosaccharides are attached to Asn through a common pentasaccharide core (3 mannose/Man, 2 N-acetylglucosamine/GlcNAc residues)
Examples of Biological Glycoproteins
Erythropoietin (EPO): A hormone glycoprotein with three N-linked and one O-linked glycan, crucial for red blood cell production.
Blood Group Antigens: Glycoproteins on red blood cell surfaces that determine blood types based on specific glycosylation patterns (all have an O antigen core, type A/B glycosyltransferases form their respective antigens)
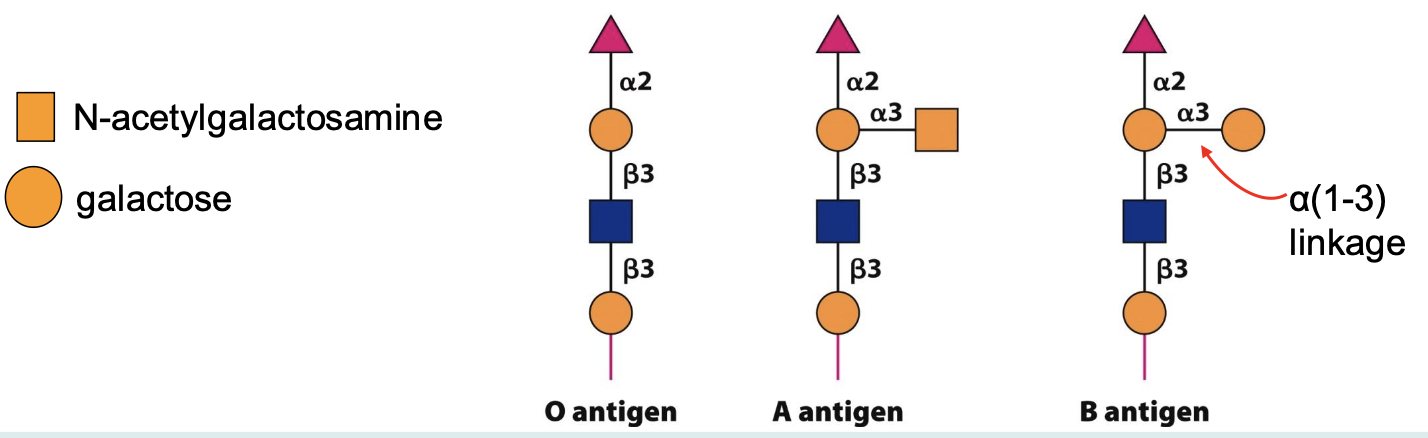
Immunoglobulins: Antibodies that contain N-linked glycans, which enhance their stability and function in the immune response.
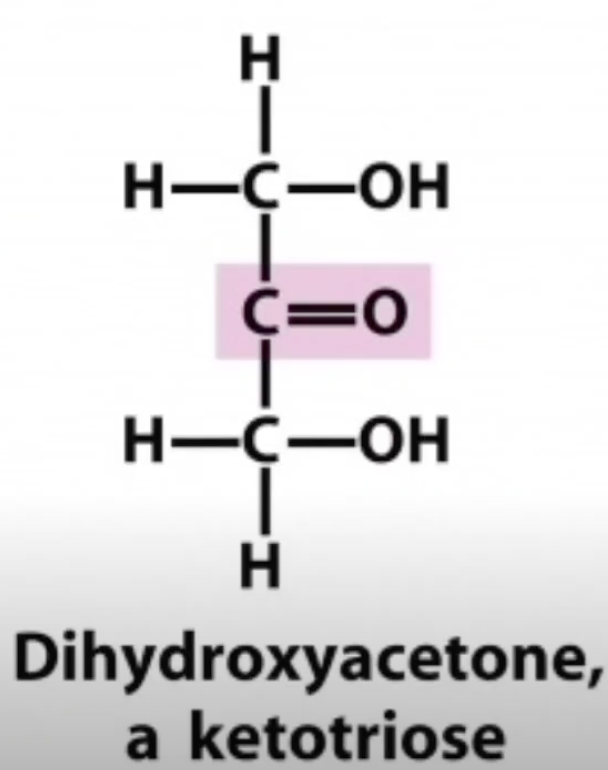
Dihydroxyacetone
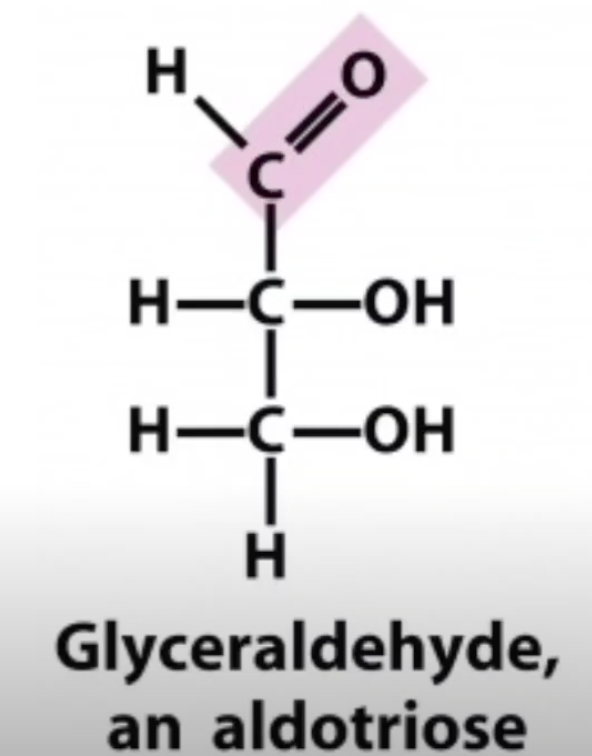
D-glyceraldehyde
D-ribose
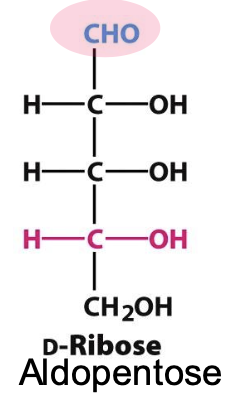
carbohydrate component of RNA
D-mannose
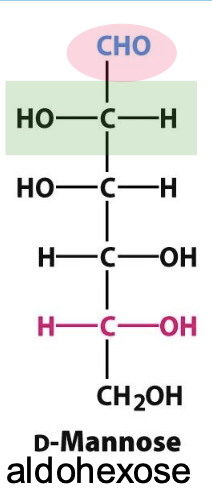
Epimer of glucose, important in the glycosylation of proteins
D-galactose
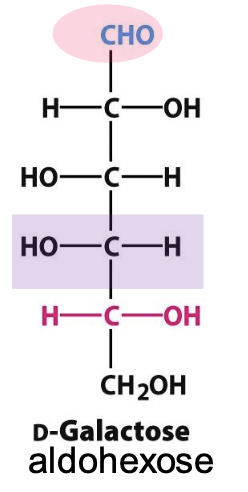
Epimer of glucose