1. General principles and classification :)
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
19 Terms
Characteristics of cancer
• Uncontrolled proliferation (autonomous)
• Dedifferentiation and loss of function
• Tissue invasiveness-metastasis
FOUR Differences between cancer and infection
Infections Involve a Biologically Foreign Microbe
Infections are caused by external pathogens (bacteria, viruses, fungi, etc.) that are not part of the host.
Pathogen Metabolism Differs from Host Cells
Microbes have distinct metabolic pathways, making them easier to target selectively.
Selective Action of Chemotherapeutic Agents
Antimicrobials can often kill/inhibit microbes without damaging host cells due to this metabolic difference.
In contrast, anticancer drugs may also affect normal dividing cells (e.g. hair follicles, GI lining).
Host Immune System Aids in Defense
In infections, the body mounts a strong immune response using:
Antibodies
Phagocytosis
Cancer often evades or suppresses the immune response, making defense more difficult.
Factors influencing tumor genesis
Gene Mutations
Core driver of cancer development.
Includes activation of oncogenes and inactivation of tumor suppressor genes.
These mutations disrupt normal cell cycle control and promote uncontrolled growth.
Hormonal Action
Certain hormones (e.g. estrogen, testosterone) can promote growth of hormone-sensitive tumors like breast or prostate cancer.
Hormones can stimulate proliferation of cells, increasing the chance of mutation.
Co-Carcinogens
These are substances that enhance the effect of carcinogens but aren’t carcinogenic on their own.
They act by promoting inflammation or interfering with DNA repair mechanisms.
Tumor Promoter Effects
Tumor promoters are agents that stimulate cell proliferation after the initial genetic mutation.
They do not cause DNA damage themselves but enhance tumor development by increasing the proliferation of mutated cells.
Proto-oncogenes:
Normal genes involved in cell growth and division.
When mutated, they become oncogenes.
Oncogenes:
Mutated proto-oncogenes that promote uncontrolled cell division and survival.
Gain-of-function mutations — only one allele needs to be mutated for effect.
Tumor Suppressor Genes:
Genes that inhibit cell growth and promote DNA repair or apoptosis.
Loss-of-function in both alleles leads to cancer progression (e.g., TP53, RB).
Types of Cancer chemotherapy
Curative Chemotherapy
Aimed at complete eradication of the cancer.
Most effective in cancers with high chemosensitivity.
Examples:
Testicular cancer
Lymphomas (e.g., Hodgkin and non-Hodgkin)
Leukaemias (especially acute types)
Adjuvant Chemotherapy
Given after surgery or radiation to eliminate micrometastases and reduce relapse risk.
Improves long-term survival.
Examples:
Breast cancer
Colon and rectal cancers
Multimodal (Combined-Modality) Therapy
Combines chemotherapy with surgery and/or radiation.
Used when cancer requires different treatment strategies for local and systemic control.
Examples:
Head and neck tumors
Lung cancer
Cervical and esophageal cancer
Sarcomas
Pediatric solid tumors
Emerging/Advanced Approaches
Involves novel therapies alongside traditional chemo:
Genetic therapy – targeting specific mutations.
Immunotherapy – manipulating immune response to attack cancer (e.g. checkpoint inhibitors).
Angiogenesis inhibition – blocking blood supply to tumors (e.g., bevacizumab).
Hematopoiesis stimulation – using agents like G-CSF to support bone marrow during chemo.
Uses of chemotherapeutic agents
Cytotoxic Anti-Tumor Therapy
Used to kill or inhibit the proliferation of cancer cells.
Examples: Methotrexate, Cyclophosphamide, Doxorubicin.
Immunosuppressive Therapy
Used to suppress abnormal immune responses in:
Autoimmune diseases (e.g., Rheumatoid Arthritis, Lupus)
Organ transplantation (to prevent rejection)
Examples: Azathioprine, Methotrexate, Cyclosporine.
Treatment of Sickle Cell Anemia
Some agents like Hydroxyurea increase fetal hemoglobin (HbF) levels, reducing sickling.
Psoriasis
Cytotoxic/immunosuppressive drugs reduce abnormal skin cell proliferation.
Example: Methotrexate, Cyclosporine.
Anti-Infective Chemotherapy
Includes antibiotics, antivirals, antifungals, and antiparasitics.
Targets pathogens selectively without harming host cells.
MOA of chemotherapeutic agents (6)
DNA Interaction & Damage
Direct interaction with DNA: Causes cross-linking, strand breaks, or interference with replication.
Example: Alkylating agents like cyclophosphamide.
Irreparable DNA damage: Triggers apoptosis in rapidly dividing cells.
Example: Cisplatin.
Inhibition of Genetic Material Synthesis
Blocks DNA or RNA synthesis, especially in dividing cells.
Example: Antimetabolites like methotrexate (inhibits dihydrofolate reductase) or 5-FU.
Anti-Proliferative Action
Targets mitosis or cell division machinery, halting proliferation.
Example: Paclitaxel (stabilizes microtubules), vincristine (prevents microtubule formation).
Immune Modulation
Enhances tumor-killing immune cells:
Example: Interleukin-2 (IL-2) stimulates proliferation of cytotoxic T cells and NK cells.
Kinase Inhibition
Inhibits tyrosine kinases that send growth signals in cancer cells.
Example: Imatinib – a tyrosine kinase inhibitor used in CML (targets BCR-ABL fusion protein).
Tyrosine kinases are enzymes that signal cell growth and survival.
Monoclonal Antibodies
Specifically target tumor antigens, leading to direct killing or immune-mediated destruction.
Examples:
Rituximab – targets CD20 on B-cells
Trastuzumab – targets HER2/neu in breast cancer
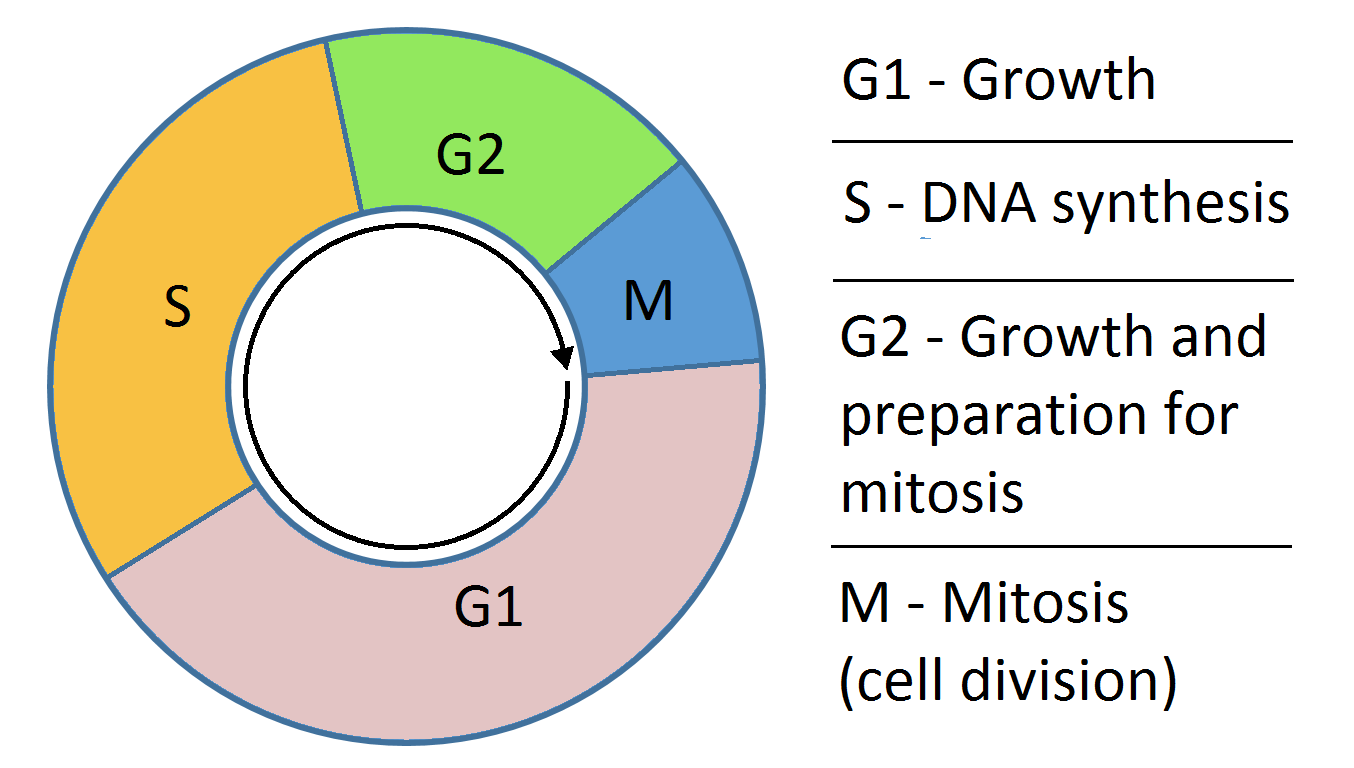
cell cycle
Cell cycle
• S phase - DNA synthesis
• G 2 phase - pre-mitotic interval
• M phase - mitosis
• G 1 phase - period between mitosis and DNA
synthesis
• Go phase - resting phase
Positive regulators of the cell cycle
• Cyclins
• Cyclic dependent kinases
Negative regulators of cell cycle
• P 53 protein
• Rb protein
• Cdk inhibitors
Chemotherapeutic regimen
• Combination therapy - synergism
• Drug interaction and toxicity
• Use drug with non overlapping mechanism of resistance and toxicity
• Maximum dose and dose interval
Survival tumor cells is mainly due to
• Loss of p53 suppressor oncogene, loss of apoptosis
• Over expression of bcl-2 oncogene that causes cell proliferation
Relationship of anti tumor drugs to cell cycle
Drug Class | Phase of Cell Cycle Affected | Examples | Mechanism |
|---|---|---|---|
Phase Non-Specific | Active in all phases including G0 | Alkylating agents, Nitrosoureas, Antibiotics (e.g. doxorubicin), Procarbazine, Cisplatin, Dacarbazine | Damage DNA regardless of the cell’s position in the cycle |
S Phase Specific | DNA synthesis | Cytosine arabinoside, Hydroxyurea | Inhibit DNA synthesis or cause faulty DNA incorporation |
S Phase Specific (Self-limiting) | S phase but with limited duration of activity | Methotrexate, 6-Mercaptopurine | Inhibit nucleotide synthesis → interfere with DNA replication |
M Phase Specific | Mitosis | Vincristine, Vinblastine, Paclitaxel | Inhibit mitotic spindle formation (microtubule inhibitors) |
🔬 Key Points
S phase is highly sensitive to drugs because of active DNA synthesis — thus toxicity (e.g., bone marrow suppression) is often greatest here.
M phase drugs (like vinca alkaloids and taxanes) prevent proper chromosome segregation → cell division arrest.
Phase non-specific drugs are useful for killing both dividing and resting cancer cells.
GIVE EXAMPLES OF CELL CYCLE NON SPECIFIC CANCER DRUGS
• Alkylating agents -
Nitrogen mustards -
mechlorethamine
cyclophosphamide,
melphalan,
chlorambucil
• Ethylenimines -
triethylenethiophosphoramide(Thio-TEPA)
• Methylhydrazine derivatives-
procarbazine
• Triazenes
dacarbazine
• Nitrosoureas -
carmustine,
bendamustine
• Platinum coordination complexes -
cisplatin
carboplatin
oxaliplatin
• Antibiotics - dactinomycin, daunorubicin, doxorubicin, plicamycin, mitomycin
GIVE EXAMPLES OF CELL CYCLE SPECIFIC CANCER DRUGS
These drugs act only when cells are in specific phases of the cell cycle, so they are most effective against rapidly dividing cells.
Drug Class | Examples | Phase of Cell Cycle Affected | Mechanism of Action |
|---|---|---|---|
Antimetabolites | Cytarabine, 5-Fluorouracil (5-FU), 6-Mercaptopurine (6-MP) | S phase | Inhibit DNA synthesis by mimicking normal nucleotides |
Peptide Antibiotics | Bleomycin | G2 phase | Causes oxidative damage to DNA, mainly before mitosis |
Podophyllotoxins | Etoposide, Teniposide | G2/phase | Inhibit topoisomerase II → DNA strand breaks |
Plant Alkaloids | Vincristine, Vinblastine, Vinorelbine | M phase | Inhibit microtubule assembly → block mitotic spindle |
Taxanes | Paclitaxel Docetaxel Cabazitaxel | M phase | Stabilizes microtubules → prevents their disassembly |
💡 High-Yield Tip:
These drugs won’t work well on non-dividing (G0) cells.
Combining cell cycle-specific with non-specific agents helps target a broader range of tumor cells.
Alkylating agents
• Cyclophosphamide
• Meclorethamine
• Melphalan
• Chlorambucil
• Ifosfamide
• Thiotepa
• Busulpan
MOA of alkylating agents
• Transfer alkyl group to various cellular constituents
• Leads to cell death
• Resistance by repairing of DNA
Nitrosoureas examples
• BCNU - Carmustine
• CCNU - Lomustine
• Methyl - CCNU -semustine
• Methyl - CCNU -semustine
Other than alkylating agents other anticancer drugs
• Antimetabolites
• Antibiotic and
• Vinca alkaloid
Anti tumor antibiotics
• Actinomycin D
• Dactinomycin
• Daunorubicin, Doxorubicin, Idarubicin
• Bleomycins