neonatology
1/41
Earn XP
Description and Tags
week 2 specialist topics in biomed
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
42 Terms
ectopic pregnancy
implantation outside of endometrial cavity (uterus) e.g in fallopian tube, ovary, pelvic peritoneum
predisposed by inflammatory diseases
conceptus invades surrounding tissue causing intraperitoneal haemorrhage- blood loss may be considerable
spontaneous foetal loss
causes:
foetal: first trimester, chromosomal abnormalities
maternal: endocrine, physical, immunological, maternal diabetes
maternofoetal: third trimester, infection (rubella, CMV, herpes, syphillis)
treatments of ectopic pregnancy
monitoring of progress
identified by declining serum hCG levels
for early stage pregnancies which are small and may self resolve
small risk of fallopian tube rupture
methotrexate
induces abortion- subsequent pregnancies during treatment need to be avoided
liver toxicity when combined with alcohol
small risk of fallopian tube rupture
surgery
excision
limited impact on fertility
anti-D rhesus needed for RhD negative mothers
gestational trophoblastic disease (GTD)
trophoblast that forms the wall of the blastocyst during foetal development
implantation of the fertilised egg into the uterine wall
5 types:
hydatidiform mole
invasive mole (malignant)
choriocarcinoma (malignant)
placental site trophoblastic tumour (malignant)
epithelioid trophoblastic tumour (malignant)
hydatiform mole
complete hydatidiform moles
contains no maternal DNA and foetal tissue
due to single spermatozoan duplication and fertilisation in an empty ovum or 2 separate spermatozoa fertilise an empty ovum
partial hydatidiform moles
contain foetal cells
triploid in origin, one set of maternal haploid genes and 2 sets of paternal haploid genes due to dispermic fertilisation of a normal ovum
foetus generally malformed and is not viable
diagnosis includes elevated serum hCG
treatment by methrotrexate, dactinomycin
more advanced cases may require radiotherapy
use of chemotherapy may advance menopause by several years
why dispermic hydatiform moles don’t develop
lack of maternal X chromosome to initiate morula and separation of chorion (placental tissue) and amnion (foetal tissue)
hydatidiform mole
beta- hCG levels, 100000 mIU/mL
serum inhibin A and activin A levels
blood cell count with platelets (anaemia and coagulability)
blood urea nitrogen, serum creatinine levels, blood type Rh factor
possible hyperthyroidism
teratogenicity
disruption to normal development of an embryo or foetus by environmental agents
spectrum of abnormalities: range from gross structural abnormalities to non-birth manifestations (growth retardation, delayed mental development, alterations in pubertal development)
gross abnormalities may halt pregnancy or produce congenital malformations
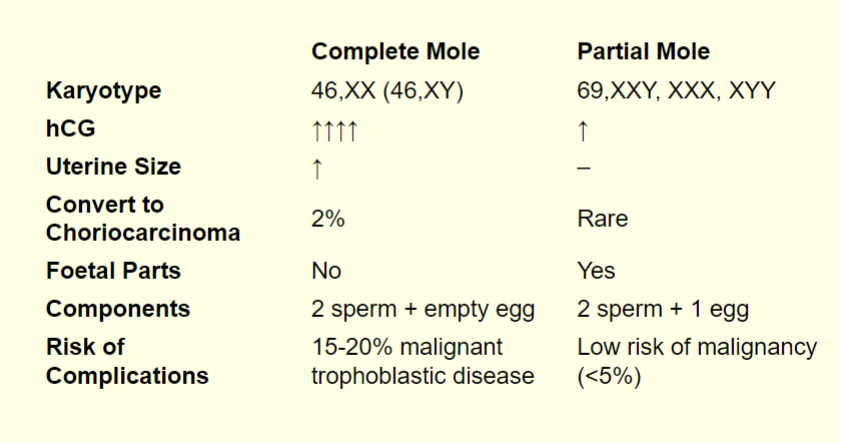
hydatiform mole table of complete mole vs. partial mole
six principles of teratology
susceptibility to teratogenesis depends on genotype of conceptus and manner in which this interacts with adverse environmental factors
susceptibility to teratogenesis varies with developmental stage at the time of exposure to an adverse influence (critical periods of susceptibility to agents and organ systems affected by these agents)
teratogenic agents act in specific ways on developing cells and tissues to initiate sequences of abnormal developmental events
access of adverse influences to developing tissues depends on nature of influence, several factors affec ability of a teratogen
four manifestations of deviant development
manifestations of deviant development increase in frequency and degree as dosage increases from the NOAEL (no observable adverse effect level) to a dose producing 100% lethality
teratology
the study of abnormalities in an organism’s physiological development , including congenital abnormalities and their causes
teratogens
mutations
chromosomal aberrations
disturbances in cell division
changes in nucleic acid composition and protein synthesis
reduction in the amount of essential constituents for biosynthesis
reduction of energy supply for embryonic and foetal development
disturbances of enzyme systems
disturbances in the regulation of water/electrolyte balance
changes in membrane characteristics
teratogens 2
toxic substances (e.g alcohol: foetal alcohol syndrome), thalomide (phocomelia), tyrosine kinase inhibitors
malnutritions
micro-organisms/infections (rubella)
physical restraint of foetus (Potter syndrome)
genetic disorders
teratoma
tumours containing normal differentiated tissues/organs (eyes, hair, bone)
generally benign
mass effect on developing foetus:
obstruction on movement of fluid through organs
competing with foetus for nutrients
may be diagnosed in utero, neonates and young children and in children and adults
graded 0-4 (only 4 is malignant)
elevated serum α- fetoprotein is biomarker for grade 4 teratomas
perinatal period
period immediately before and after birth
20th-28th week of gestation and ends 1-4 weeks after birth
adaptations to extra-uterine life from aquatic environment
physiological systems not developed yet (immune system)
pre-eclampsia
onset of hypertension at 20 weeks
8% of pregnancies
continuum of severity- mild pre eclampsia may be relatively symptomless but needs monitoring
due to placental malfunction
risk factors: family history, obesity, age, multiple babies
viability
point at which the foetus becomes viable- potentially able to live outside mother’s womb albeit artificial aid
age of viability appears to be between 23 and 24 weeks of gestation corresponding to foetal lung development
respiratory system is last organ system to achieve functional maturity
clinical complications of prematurity
cerebral palsy: 25% of extremely premature infants (extremely low birth weight)
neurosensory deficits
mental retardation
severe growth disturbance
epilepsy
chronic lung disease
surviving infants with severe handicap according to gestational age and birth weight
survivability
correlates with gestational age
50% of babies born at 24 weeks
70% of babies born at 25 weeks
90% of babies born at 27-28 weeks
neonatal mortality rate improves with each extra gestational week achieved at delivery
mortality rate at 33 weeks is very low: comparable to low mortality rate of babies born at term
preterm birth
associated with many changes:
thermoregulation
hypoglycaemia
hyperbilirubinaemia
fluids and electrolytes
apnoea of permaturity
anaemia of prematurity
fluids and electrolytes
preterm infants have proportionally more fluid in EC compartment than in IC, making them more susceptible to free water loss
disturbances of fluid balance contributes to IV haemorrhage and patent ductus arteriosus
frequent problems are hyperkelemia, hyponatremia and contributes to kidney failure
immature renal function: decreased ability to excrete excessive water loads
poorly keratinised skin
common neonatal complications
neonatal malignant neoplasms
genetic disorders
trauma
intrauterine complications
intraventricular haemorrhage
anaemia of prematurity
occurs between 2nd and 3rd month of life in term infants
occurs earlier and is more severe than in term babies
immature erythropoiesis
decreased survival of RBCs in premature infant
deficiences of folate, vit B12, iron
sudden unexplained infant death (SUID)
pulmonary oedema is commona nd non specific in SUID cases
neonatal pulmonary oedema
increased pressure in microcirculation of lungs
cause of respiratory distress in newborns
may be due to:
severe perinatal asphyxia
heart failure
hyaline membrane disease
persistent patency of ductus arteriosus
pneomonitis from group B
chronic lung disease
neonatal diarrhoea
6.72 per 1000 hospitalised newborn
infection: virus, bacteria, parasite
food allergy or sensitivity to medicines
drinking too much
poisoning
neonatal jaundice
free bilirubin crosses blood brain barrier in neonates
injury to developing bran, hearing impairment, cerebral palsy etc
hyperbilirubinaemia
80% of preterm infants develop significant jaundice requiring treatment
increased breakdown of foetal red cels
immaturity of biliary excretory function of liver
reduced bowel motility with increased enterohepatic circulation
coexisting sepsis
risk of kernicterus
respiratory distress syndrome (RDS)
hyaline membrane disease
appearance develops between 12-24 hours of life
lungs are airless, congested, liver-like consistency
basophilic debris of necrotic epithelium present in early phase
thick eosinophilic hyaline membranes (consists of necrotic bronchiolar epithelium and fibrin) lining the respiratory bronchioles and alveolar ducts in developed phase
collapse of a lung
reparative changes occur in survivors by 48 hours by phagocytosis of membranes, regeneration of epithelium and mild fibrosis
RDS 2
surfactant synthesised by type II pneumocytes
consists of lecithin, sphingomyelin and surfactant associated proteins
reduces surface tension at air air-liquid barrier in alveoli
produced in considerable amounts after 35 weeks of gestation but modulation by variety of stimulation is possible
increased incidence of RDS than expected for gestational age: acute caesarean section before onset of labour, asphyxia, infants of diabetic mothers
decreased incidence of RDS than expected for gestational age: pre eclampsia, recurrent vaginal bleeding
significantly higher risk of RDS in second as compared to first of twin pairs
neonatal hepatitis
onset between 1-2 months after birth
liver inflammation due to viral infection during pregnancy or shortly after birth
enlarged liver and spleen, jaundice, mal-adsorption of vitamins, poor weight gain
may progress to liver cirrhosis and mental retardation
hepatitis A, B, C also implicated
liver cirrhosis will require transplant
diagnosis includes biopsy and blood tests
necrotising enterocolitis (NEC)
NEC more prevalent in premature infants but can also be observed in near-term or term infants
in preterm infants the incidence is inversely related to gestational age
NEC in term infants:
initiating event: ischaemic insult to gut frequently following birth asphyxia
NEC in premature infants:
associated with enteral feeding, not with birth asphyxia
higher incidence in patent ductus arteriosus
average age of onset in preterm babies is the 2nd/3rd week of life
rubella (German measles)
togavirus, enveloped, single stranded RNA genome
infection causes physical deformity, cardiac, cerbral ophthalmic, and aditory defects
maternal infection in first 12 weeks of pregnancy
teratogenic mechanism currently unclear but may include necrosis, apoptosis, mitochondrial abnormalities, cytoskeleton disruption
immunisation and screening of teenage girls before pregnancy
lack of compliance with vaccination programmes
post-partum thyroiditis
missing or poorly developed gland
faulty pituitary gland
inappropriate thyroid hormone production
causes:
pharmaceutical medication during pregnancy
insufficient maternal dietary iodine
inappropriate thyroid function due maternal antibody activity
diagnosis:
heel-prick blood test to screen for hypothyroidism shortly after birth
treatment:
levothyroxine, cheap and simple
prenatal testing
invasive:
amniocentesis
chorionic villus sampling