6) Chemical fractionation, element partitioning, geochemical classification of elements, chemical differentiation of earth
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
what is chemical fractionation
changing the mixture by doing a process where some elements are preferentially used over another
this happens with all chem rxns, there are rarely ones that use elements equally
explain chem frac during weathering, what is the formula
weather intensity differs in different layers, more intense near the top
aluminum is not easily dissolved in fluids, therefore not affected by weathering very much
increases in percentage from original to saprolite (in situ altered rock) is because we removed some other elements, making the proportions different
adjusted percent is saprolite column*0.6, then loss/gain is adjusted - original rock
explain chemical fractionation during crystallization
composition of the olivine has different composition of oxides than the original basaltic melt
it takes less silica from the melt, preferentially takes a lot of Mg from the melt, the melt will be depleted in Mg after this
what are key points in chemical fractionation in nature?
change in the chemical composition of a mixture/reservoir/material due to unequal processing of elements
can use the understanding of how elements/isotopes are preferentially used to model geological processes
how do we quantify the fractionation of elements in minerals/rocks?
partition coefficient: kd
measure of an affinity of a mineral to take a specific element into its structure
can be determined empirically by analysis of real samples, or from exprts
what is kd for mineral-mineral in numbers and explanation?
kdi: conc of i in mineral A / conc of i in mineral B
Kdi > 1: element i is more compatible in mineral A (top)
Kdi < 1: element i is more compatible in mineral B (bottom)
kdi = 1: equal distribution, element does not have a preference
what does it means for an element to be more compatible in A
the ionic radii and charge better fit into the cryst sites of A
what does incompatible element mean? what does compatible melt?
kd <1: element prefers to partition into the melt
kd >1: element prefers to partition into the mineral
compatibility is defined with respect to the mineral
what are factors that control Kd
temp, pressure, comp of mineral, comp of magma
what is the bulk partition coeff? words, math, categories, what does that mean?
used when element is simultaneously partitions between multiple minerals
Di = WA KdiA + WB KdiB (sum of the weight fraction of the mineral * partition coef of element i)
D >1: compatible, goes into mineral
D <1: incompatible, goes into melt
if La is incompatible with this peridotite, not much of it will crystallize as it solidifies. If it’s melting, more of it will stay in the melt
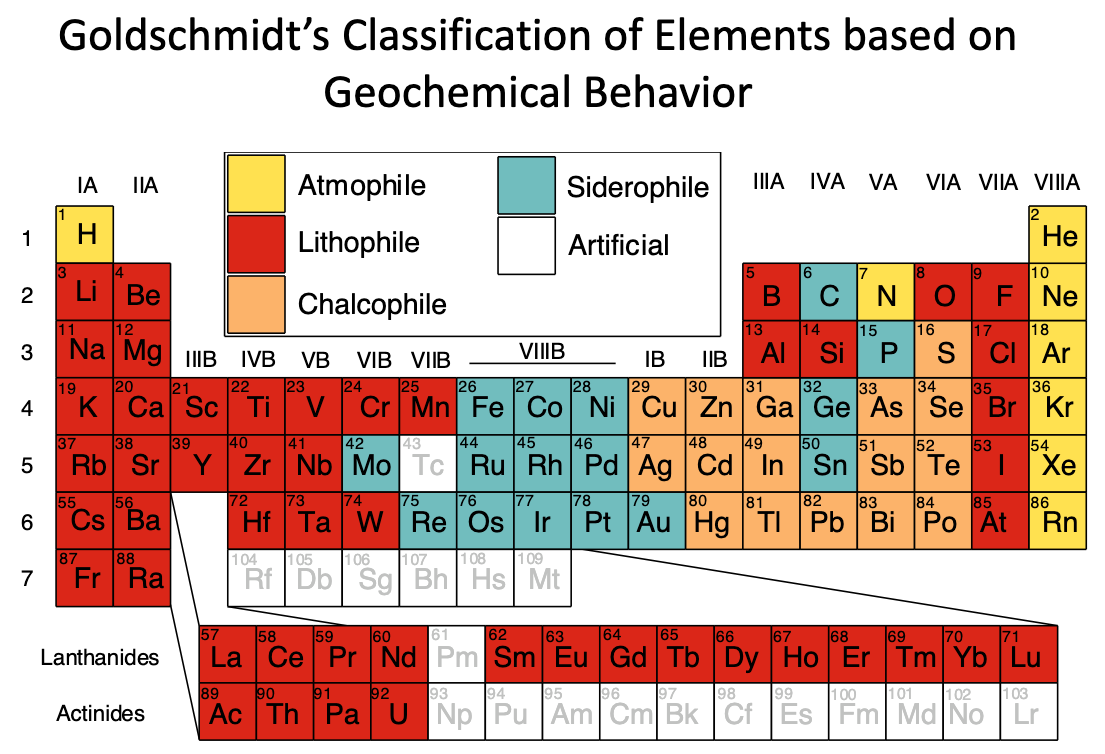
what are the 4 groups of distribution of elements by partitioning behaviour
atmophile: gas, volatile
lithophile: silicates, easily bond with oxygen (elements that become cations bond with oxy)
chalcophile: sulfide, bond with sulfur
siderophile: metallic phase
what goldschmidt classification are noble gases? halogens, alkali, alkaline? oxygen?
noble gas: atmophile, they don’t form bonds
red: lithophile, usually abundant in rocks
orange: chalcophile
blue: siderophile (metallic, middle of transition metals)
oxygen is in atmosphere as gas, but is also in many rocks
what is the composition of the crust?
oceanic: 50% silica, more than mantle, low in Mg (7.5%) continental slightly lower
continental: 60% silica, low in Mg (4.7%)
rich in aluminum, crust has measurable alkali element, mantle is negligible
what is the composition of the mantle
45% silica, typically ultramafic rocks
38% MgO, because olivine, other mafic minerals are high in Mg
we don’t have many mantle samples (apart from peridotite and ocean floor along faults)
what is the composition of the core
no samples
estimated similar comp to iron meteorite, iron-nickel alloy
we know core exists because seismic wave
what are the chemical differences of the layers of the earth (goldschmidt groups)
crust: lithophile dominant, chalcophile
mantle: lithophile dominant (Olv, Cpx, Opx, silicates), chalcophile
core: siderophile dominant, chalcophile
atmophiles are in atmosphere and hydrosphere
explain the chemical differentiation of the earth
first thing was core formation
core formed through metal silicate differentiation: core became rich in metals, and the rest became silicate rich (rest is primitive mantle, all silicates in this layer)
crust then formed from extraction of crust from the mantle (process is still happening today
explain core formation
gravity settling of dense Fe-Ni metal through less dense silicate
magma separation, gravity and density differentiation
happened in magma ocean (top layer was super hot magma ocean)
explain oceanic crust formation
formed in spreading zones in mid-ocean ridges, mantle magma upwelling
higher pressure means higher temp needed to melt it, BUT if you are upwelling, you stay at the same temp but lowering pressure, causing melting. this produces basalts
also happens at hot spots
when basaltic magma crystallizes, you produce oceanic crust, from primitive mantle, some got extract to produce oceanic crust
explain composition of oceanic crust
rich in silica, aluminum, and alkali than mantle, lower in magnesium
silica is incompatible because the melt (which is the crust) gets enriched in silica compared to the source rock (mantle) that melted
Mg is compatible because source rock (mantle) is richer in Mg compared to melt (crust)
explain composition of continental crust
very silica rich
impossible to melt mantle to cause that degree of richness from just the first stage of melting of the mantle
earth is only planet in solar system with continental crust because others dont have plate tectonics so they can’t recrystallize and remelt
needs to form oceanic crust, then re-melt it to increase the silica even further
what is the significance of element partitioning?
occurs during geologic processes such as melting, crystallization, weathering, evaporation
can be quantified by measuring the partition coeff, which can provide a fingerprint of the processes and can be used to model the processes