DP Chemistry Unit 10 Organic chem: Part 1
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
24 Terms
alcohol functional group
hydroxyl group (-OH)
aldehyde/ketone functional group
carbonyl group (C=O)
carboxylic acid functional group
carboxyl (COOH)
Benzene ring attached to a parent compound
phenyl group (-C6H5)
amide functional group
-CONH2
amine functional group
R-NH2
ether functional group
alkoxy (R-O-R')
empirical formula
The simplest whole number ratio of atoms of each element present in a compound
Full structural formula
Shows all of the bonds between the atoms of a molecule.
condensed structural formula
structural molecular formula showing the general arrangement of atoms but without showing all the covalent bonds
skeletal formula
the simplified organic formula, leaving just a carbon skeleton and associated functional groups
molecular formula
A chemical formula that shows the number and type of atoms in a molecule, but not the arrangement of the atoms.
functional groups
chemical groups attached to carbon skeletons that give compounds their characteristic physical and chemical properties
ester functional group
RCOOR
halogeno functional group
R-X
saturated molecule
A molecule that only has single carbon-carbon bonds.
unsaturated molecule
contains at least one double or triple bond
homologous series
A series of organic compounds having the same functional group, same general formula but with each successive member differing by CH2
structural isomers
molecules that have the same molecular formula but different connectivites
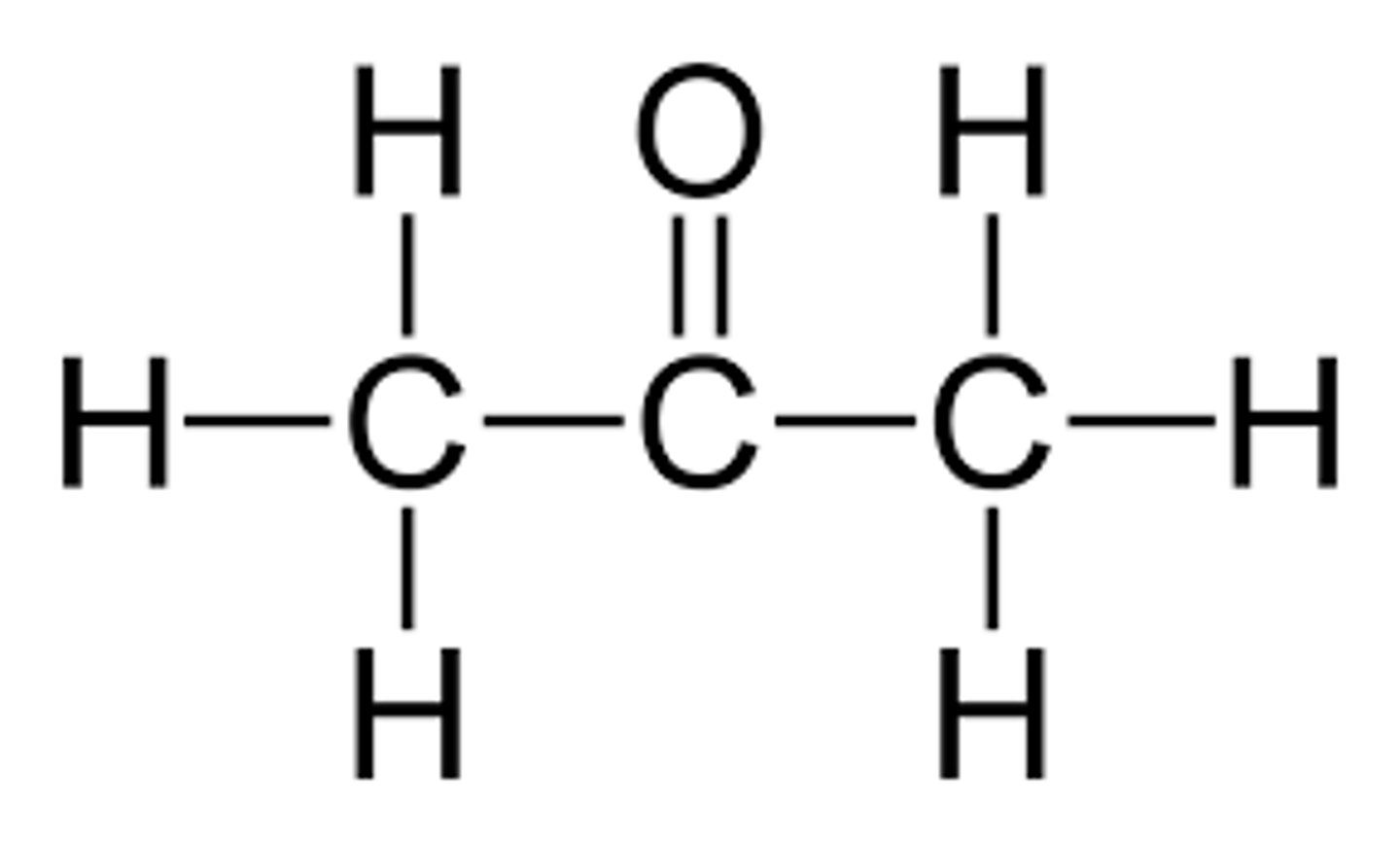
methylbutane
an isomer of pentane
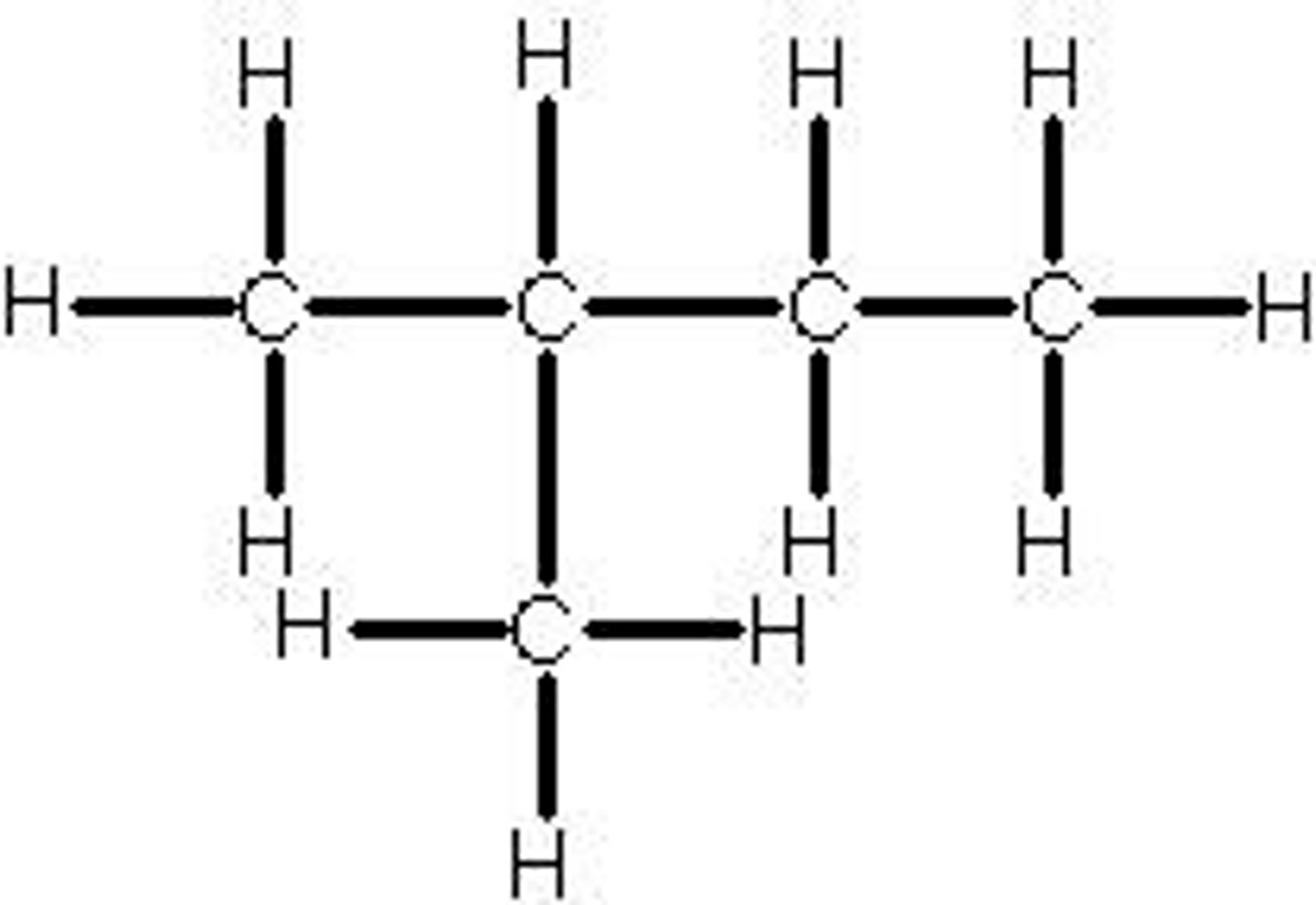
propan-2-ol
a secondary alcohol with 3 carbons
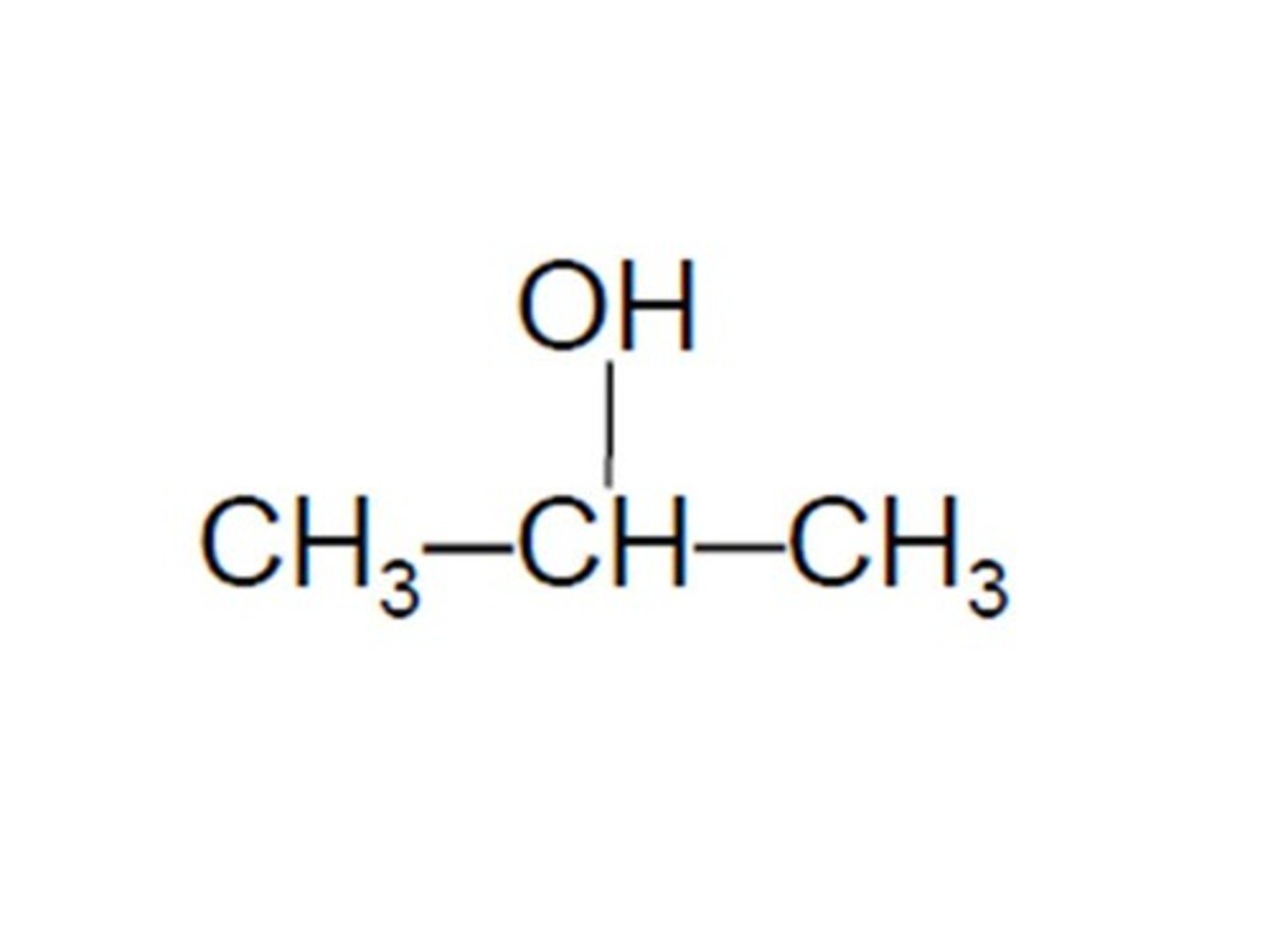
methanoic acid
the smallest carboxylic acid
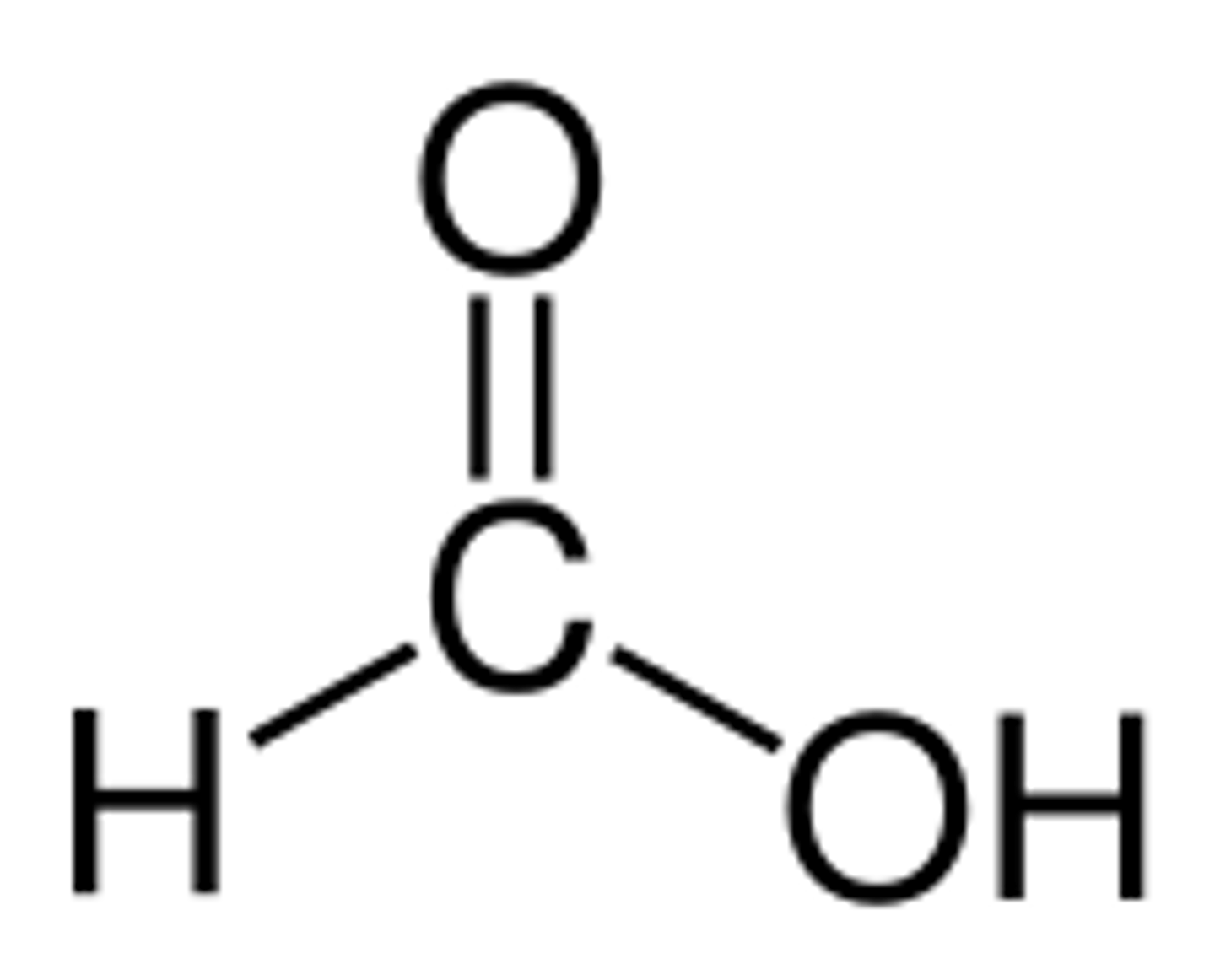
ethanal
an aldehyde with 2 carbons
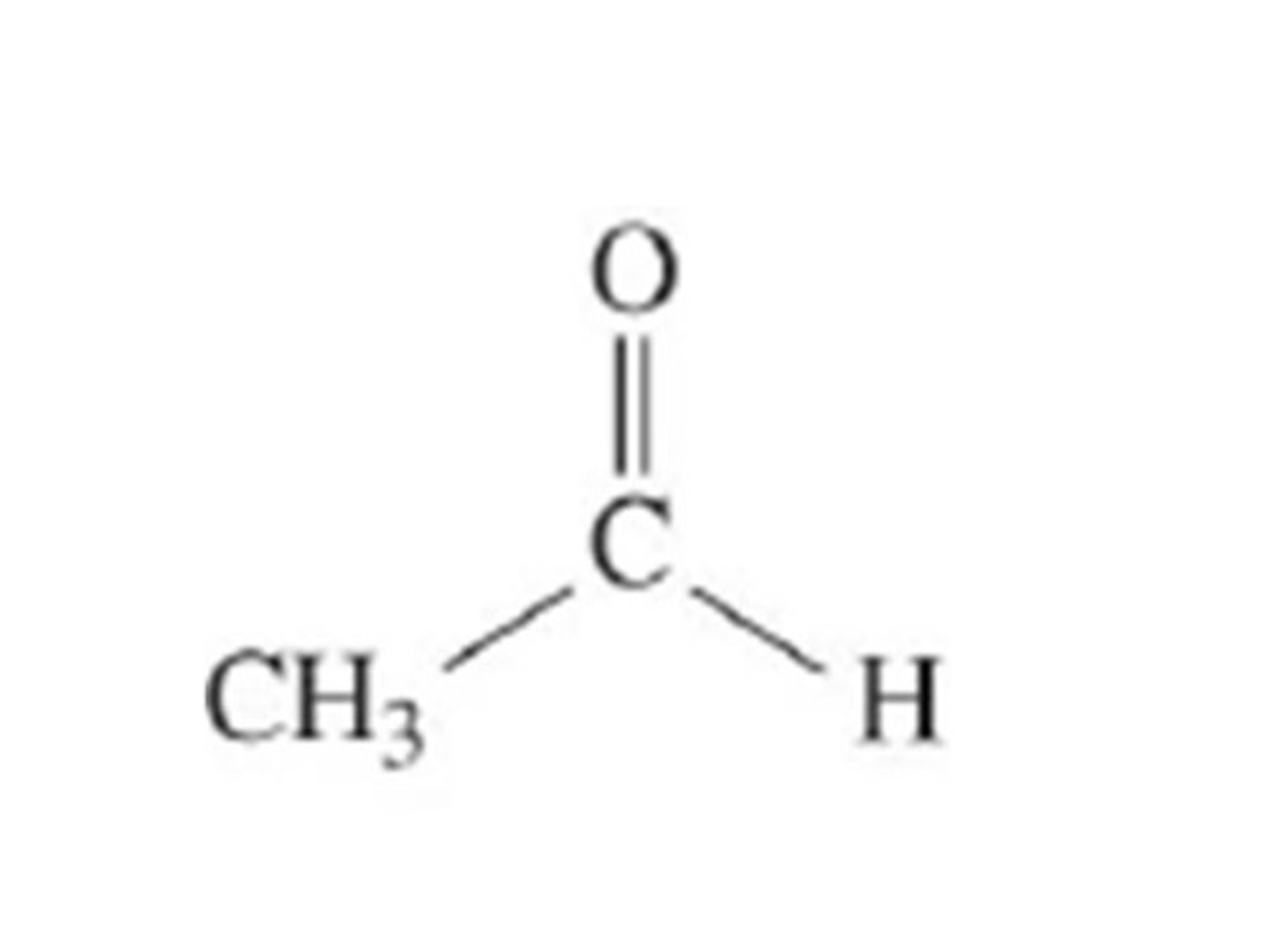
propanone
the smallest ketone