Organic Chemistry (Reactions)
1/150
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
151 Terms
Free Radical Halogenation
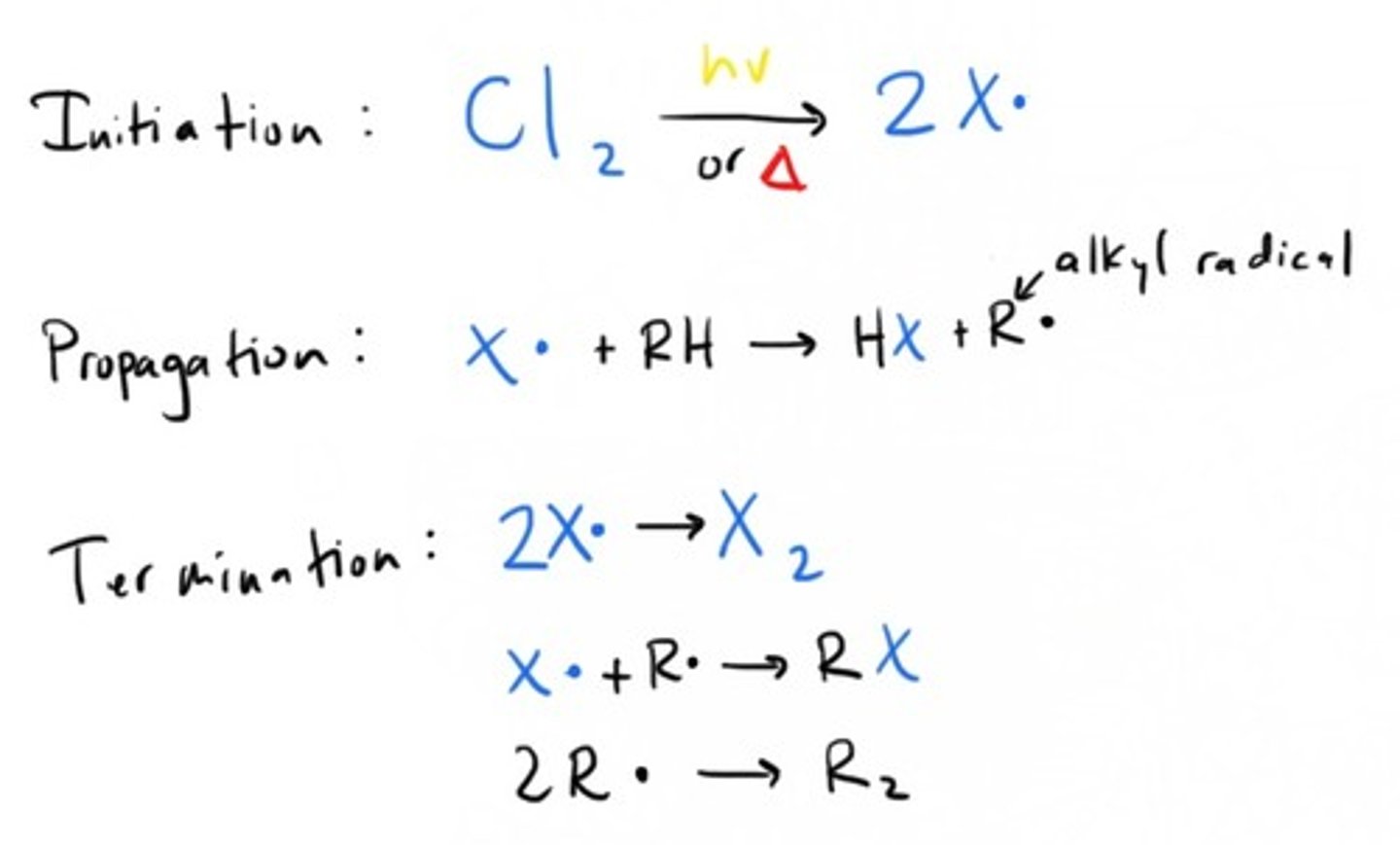
What free radical is most is likely to be formed from bromination?
A tertiary radical (Most stable)
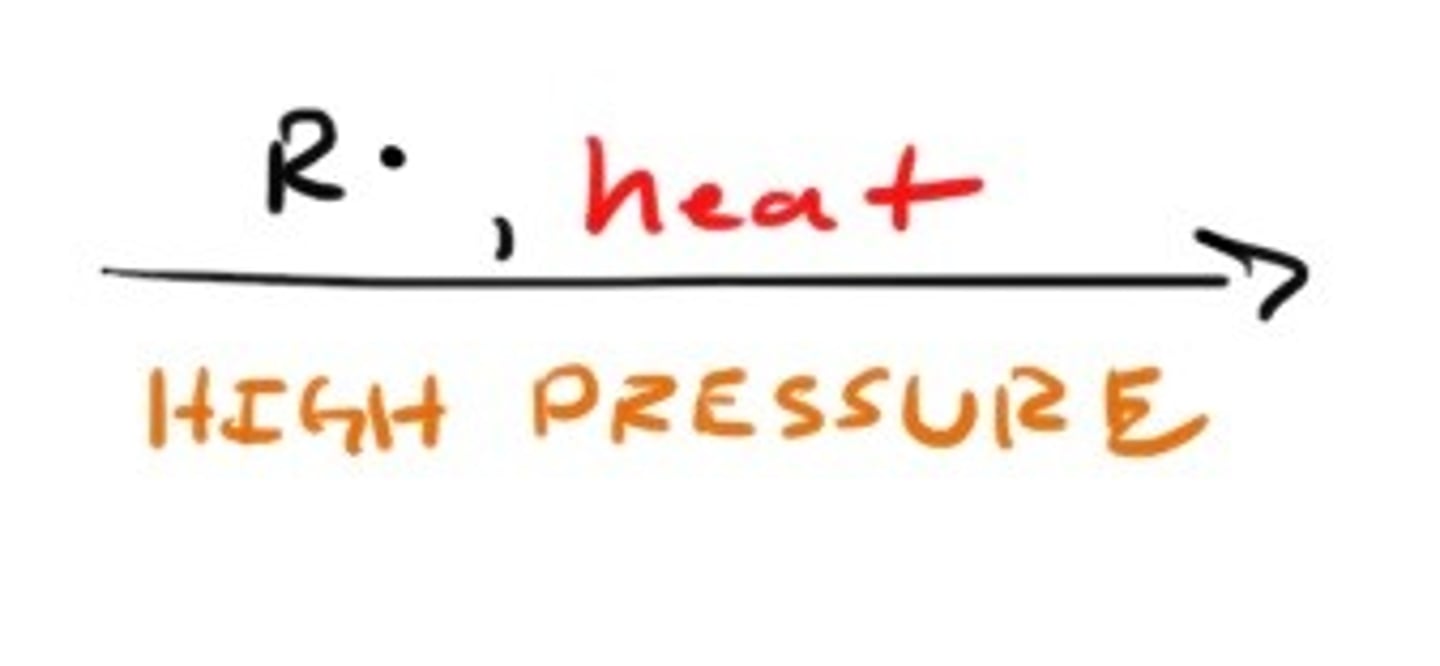
Combustion
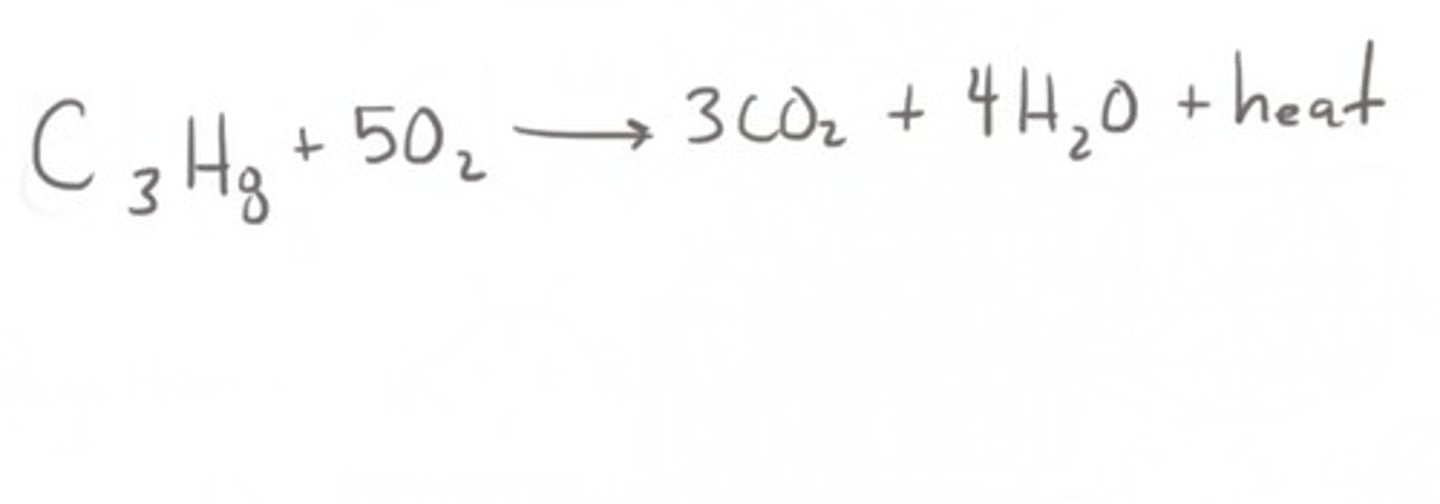
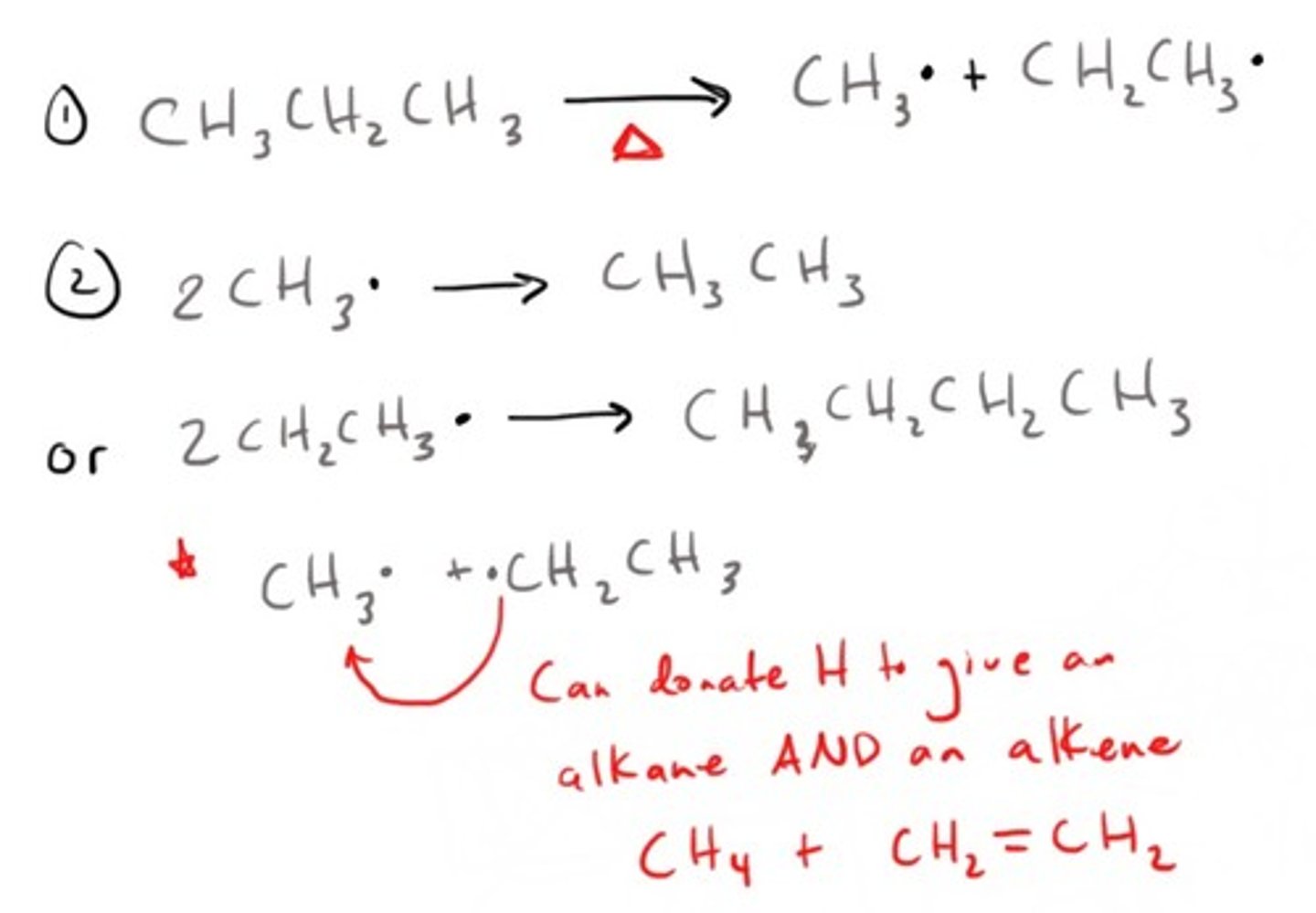
Pyrolysis (Cracking)
What are nucleophiles?
Electron-rich species that are attracted to a positively polarized atom (i.e. a carbonyl carbon)
What is a better nucleophile I or F?
Flourine
What is a better leaving group I or F?
Iodine
Sn1 Reaction
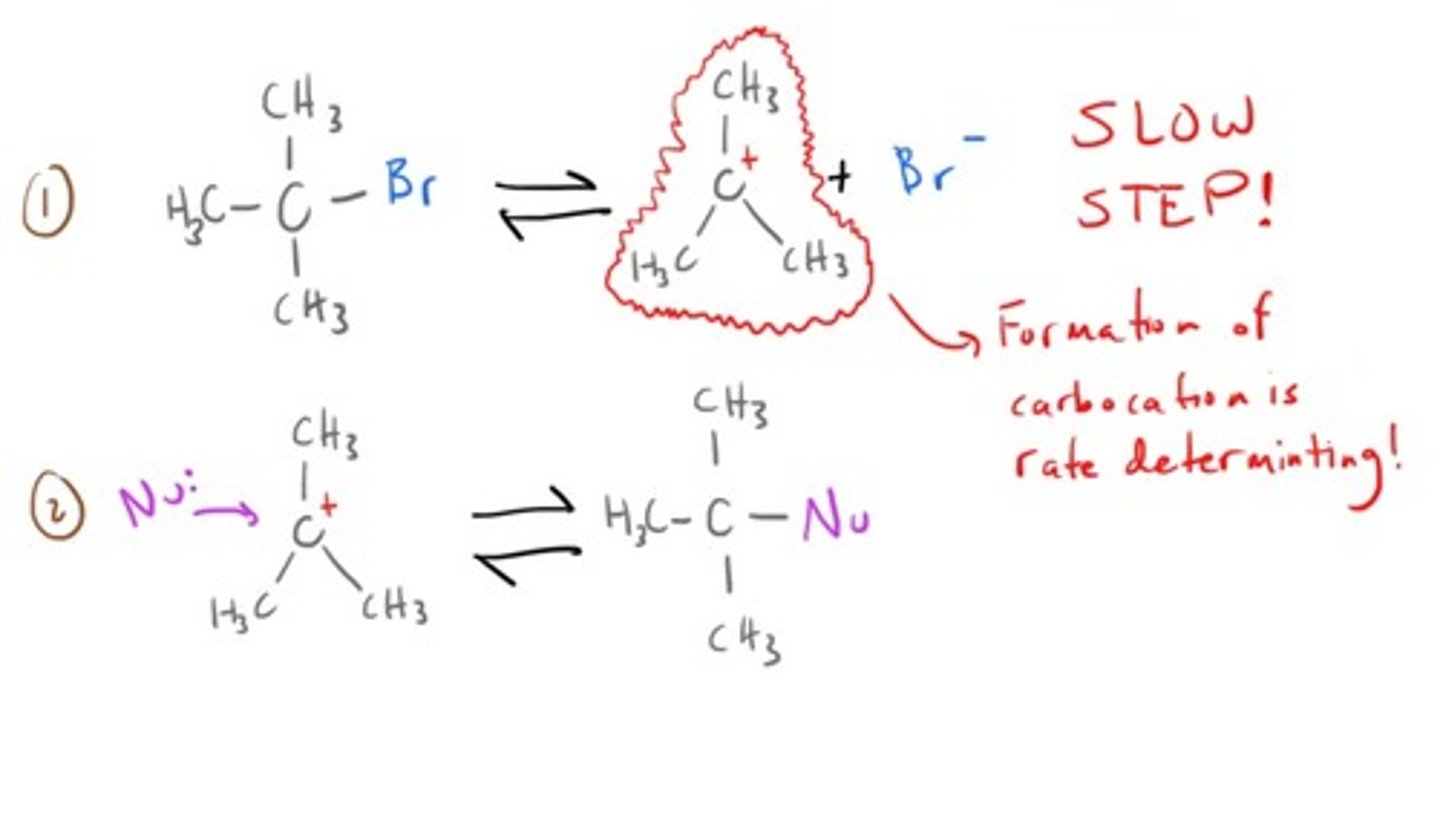
What doesn't a nucleophile play a role in the rate of an Sn1 reaction?
It is not involved in the rate-determining step...the formation of the carbocation.
What factors will help carbocation formation and therefore increase the rate of an Sn1 reaction?
1. Highly substituted carbons.
2. Polar solvents (Surround and isolate the carbocation)
3. Good leaving groups (Weak bases)
Sn2 Reaction
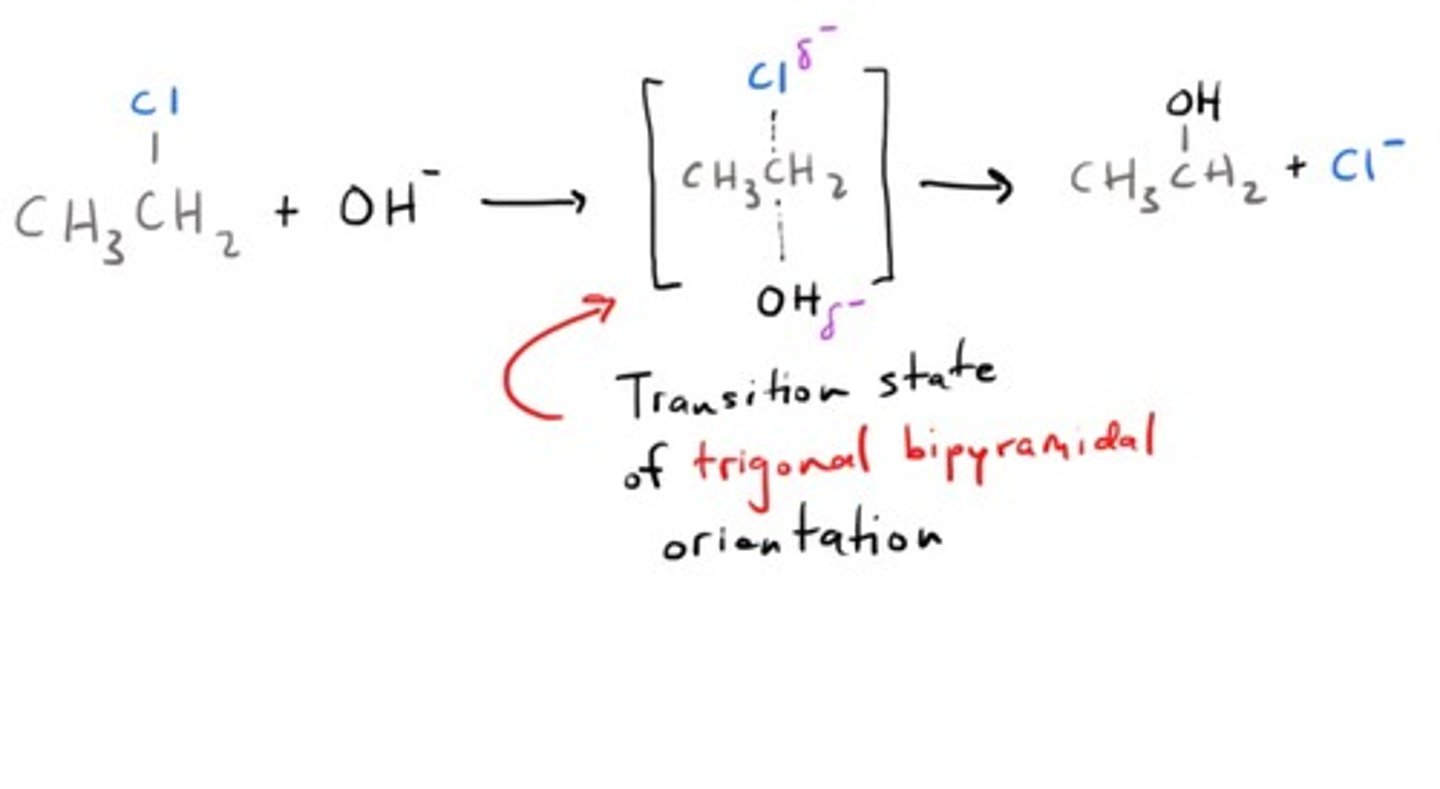
Why is a Sn2 reaction bimolecular?
Its rate-determining step is dependent on TWO species; both the substrate and the nucleophile.
What are the best conditions for an Sn2 reaction?
You must have a strong nucleophile for the backside attack (thats what she said) with no steric hindrance.
With respect to optical activity, what do your end products of Sn1 and Sn2 reactions become?
Sn1 = racemic mixture (nucleophile can attack from either the top or the bottom of the planar carbocation), therefore loss of optical activity.
Sn2 = inversion of configuration and remains optically active.
What role does tosylate usually play in reactions?
It usually acts as a good leaving group and a good protecting group.
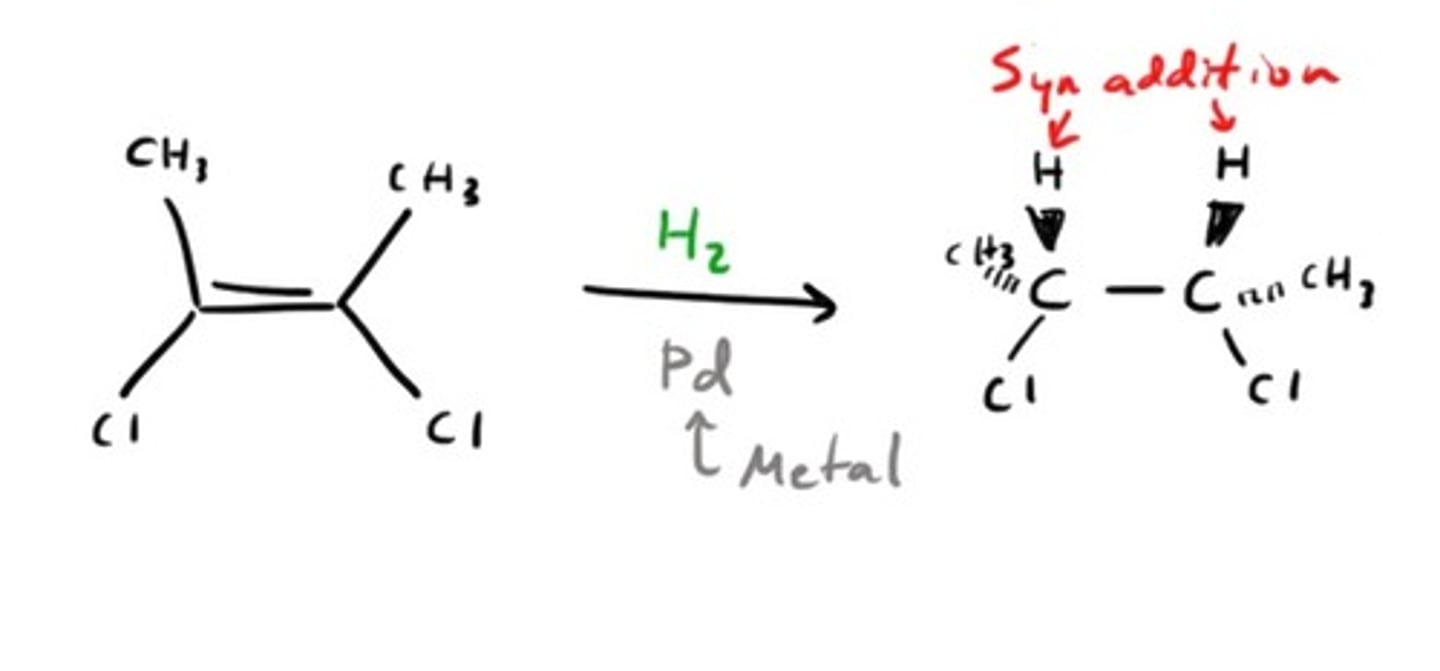
Catalytic Hydrogenation
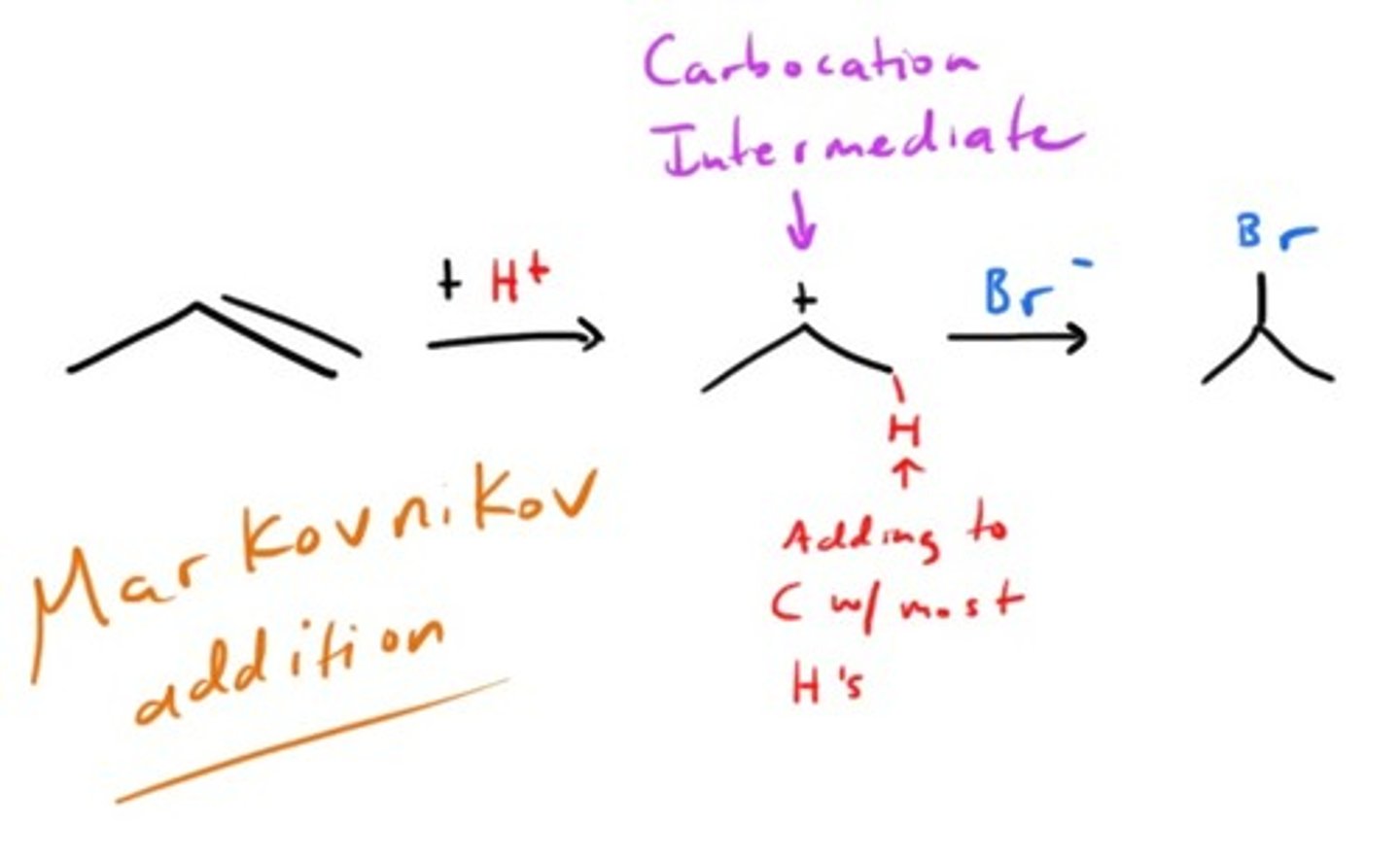
Addition of HX to an alkene
Addition of X2 to alkene
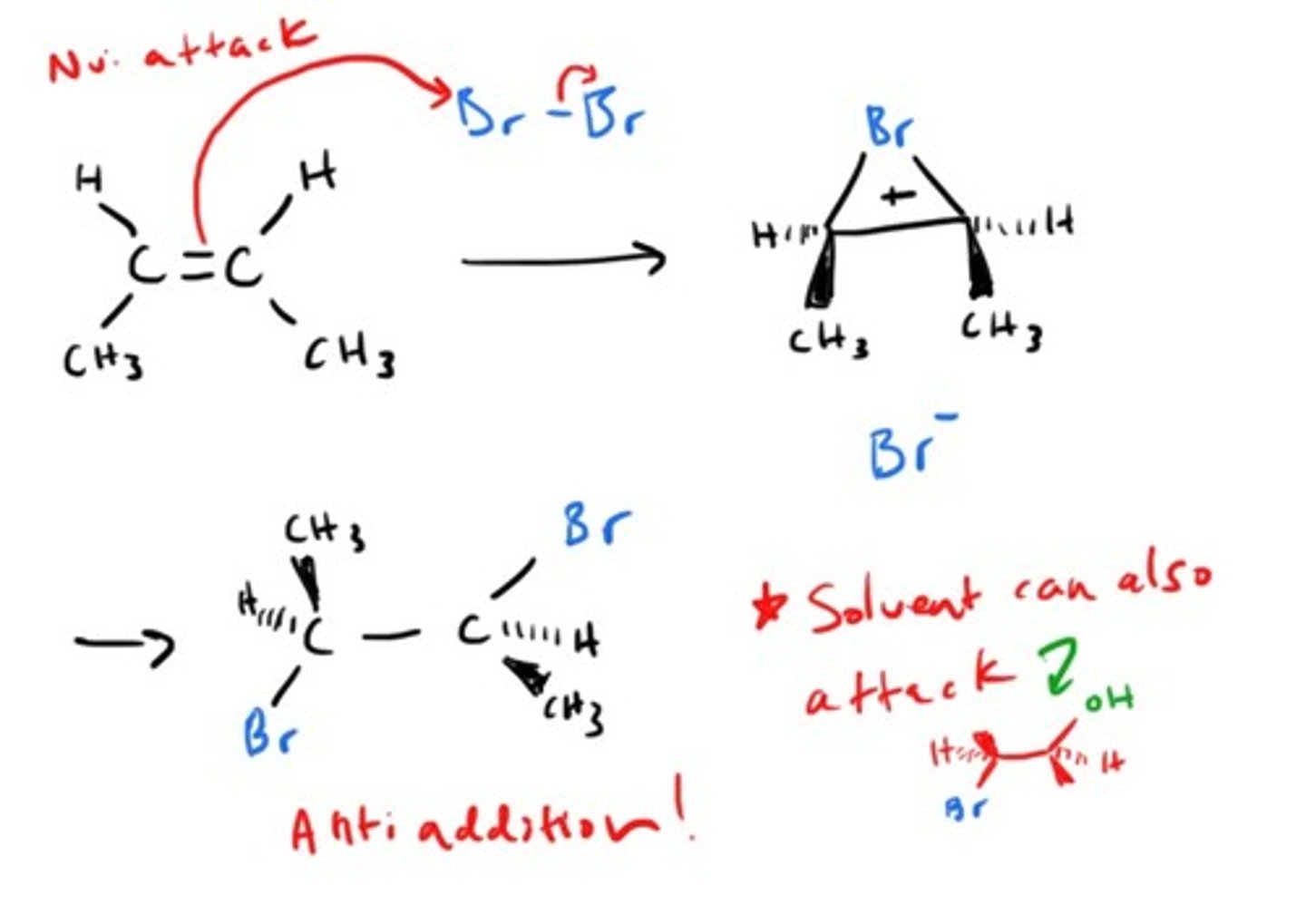
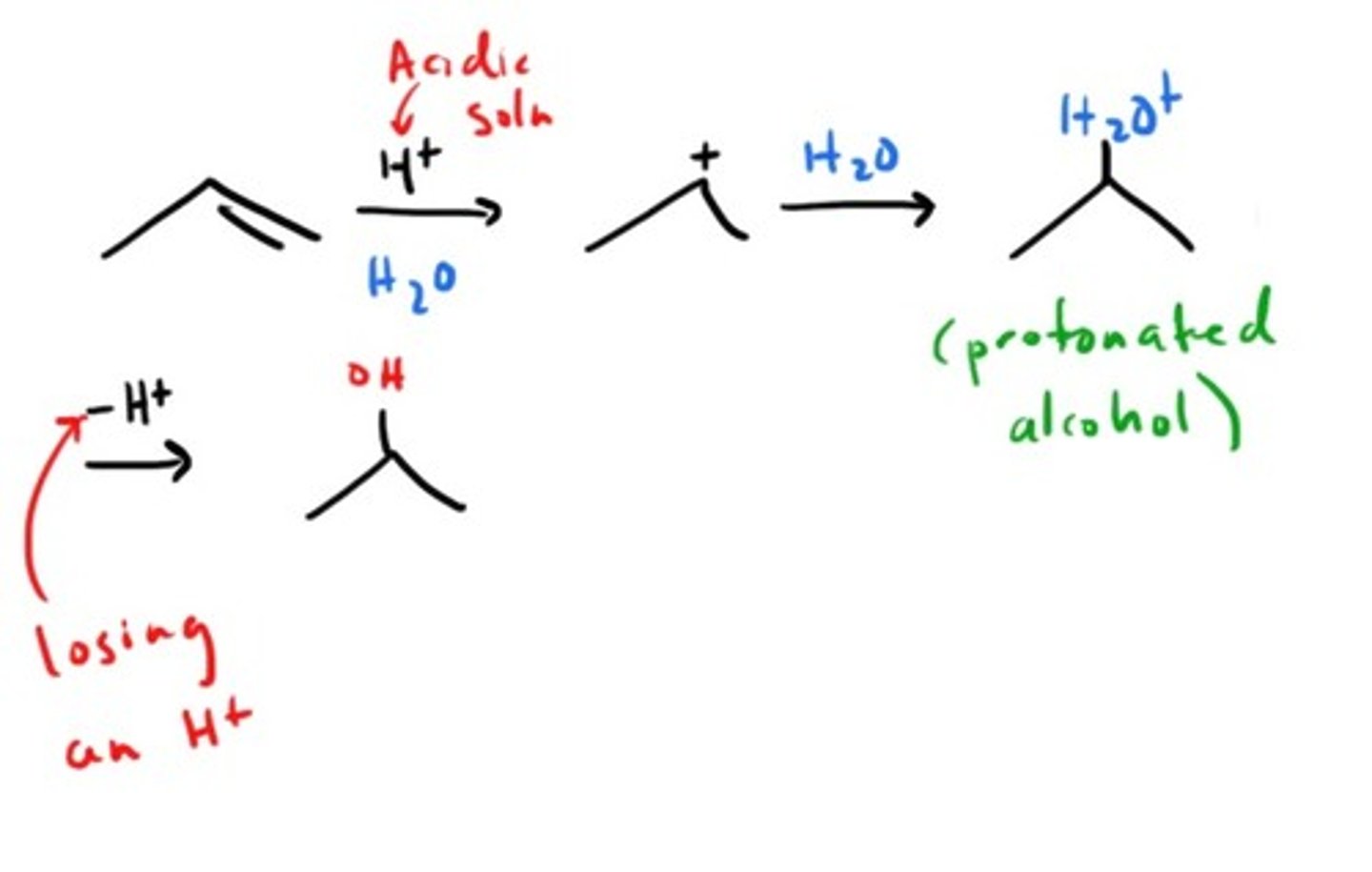
Addition of H2O to alkene
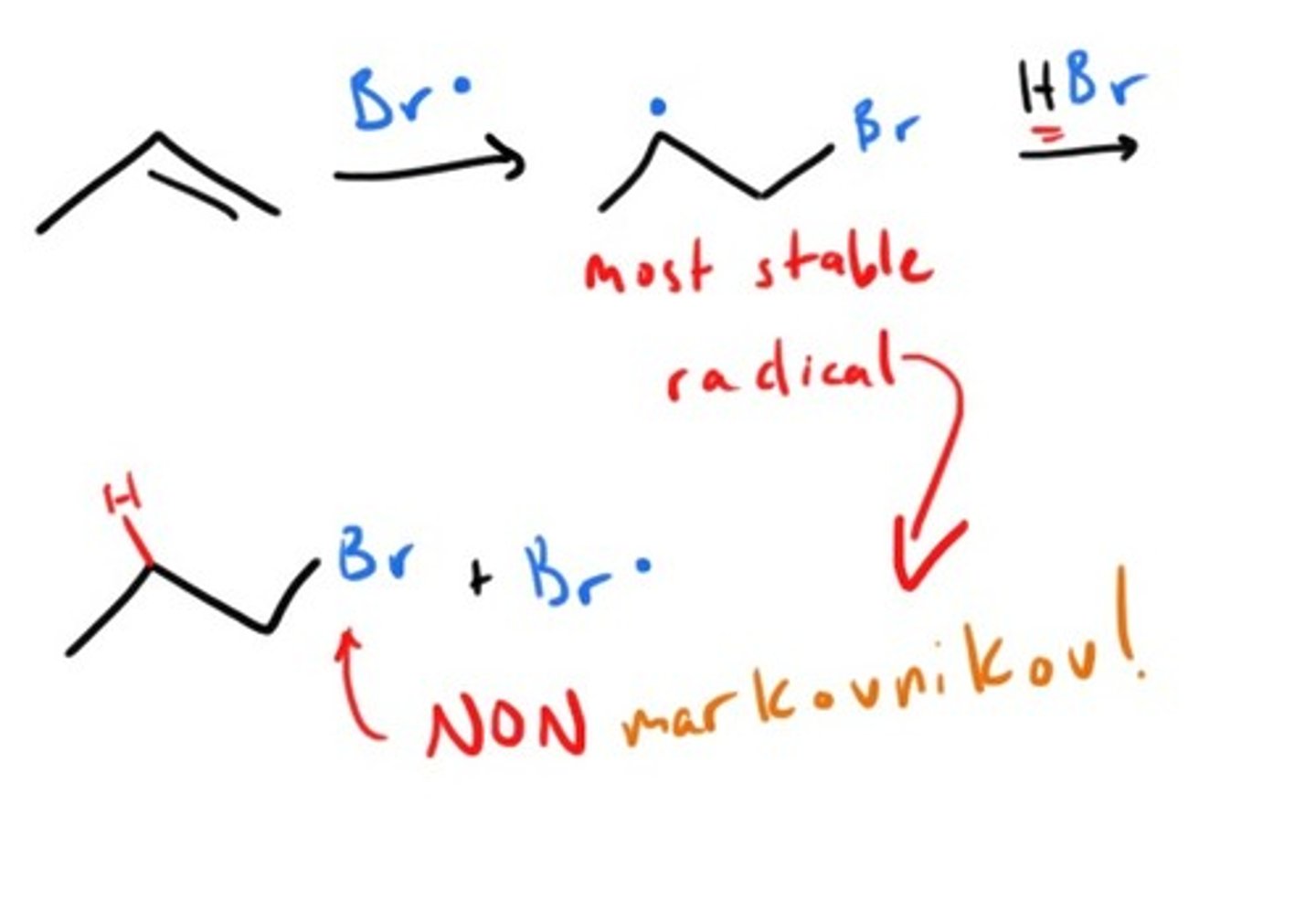
Free Radical Addtion to alkene
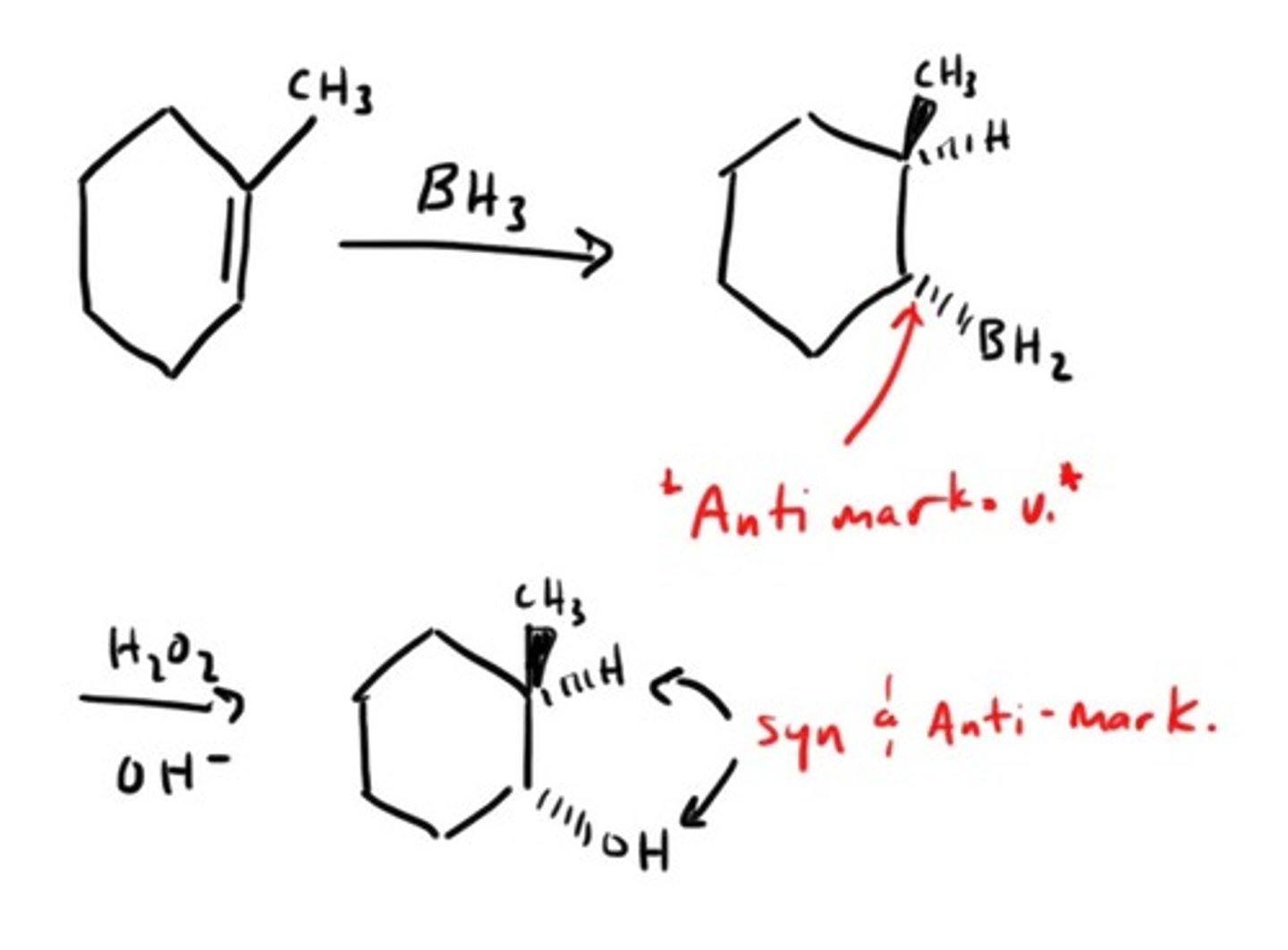
Hydroboration
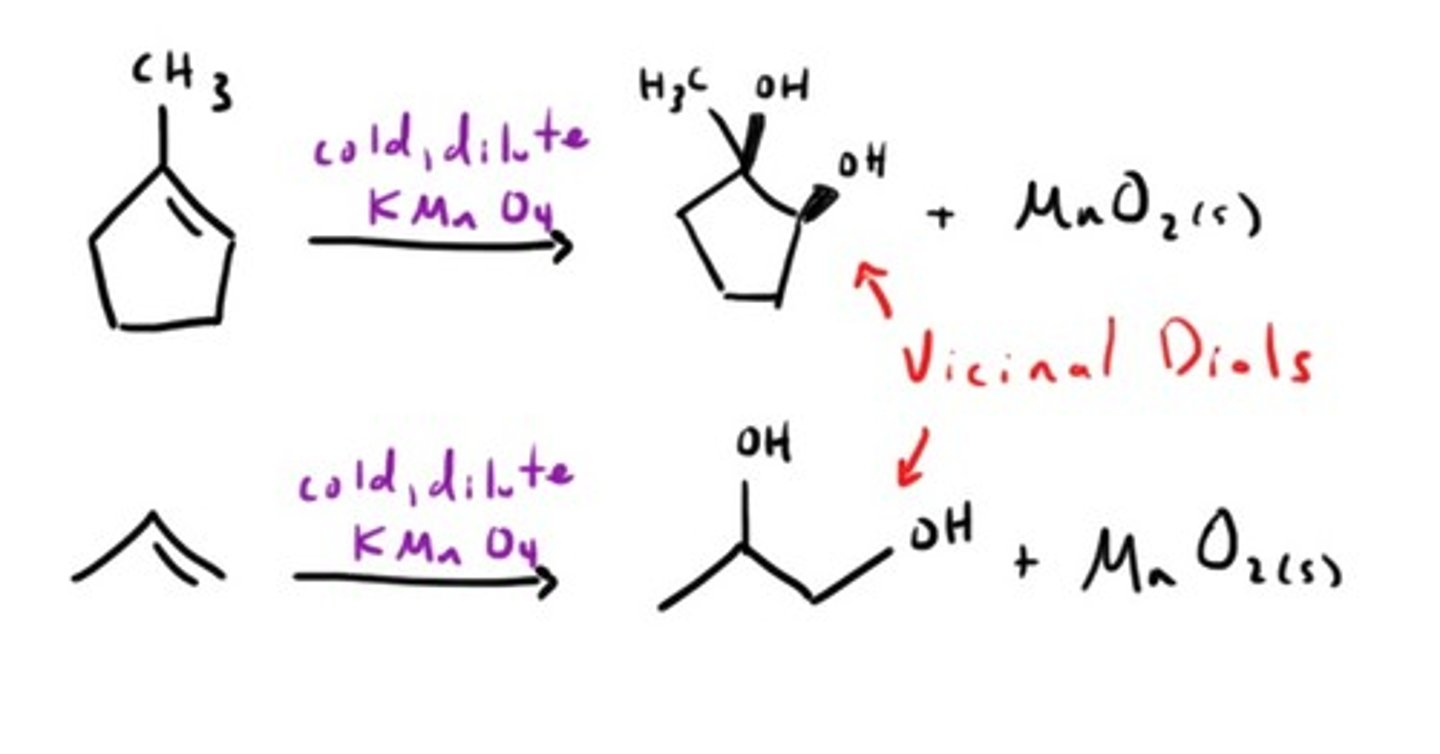
Oxidation with KMnO4 (Cold)
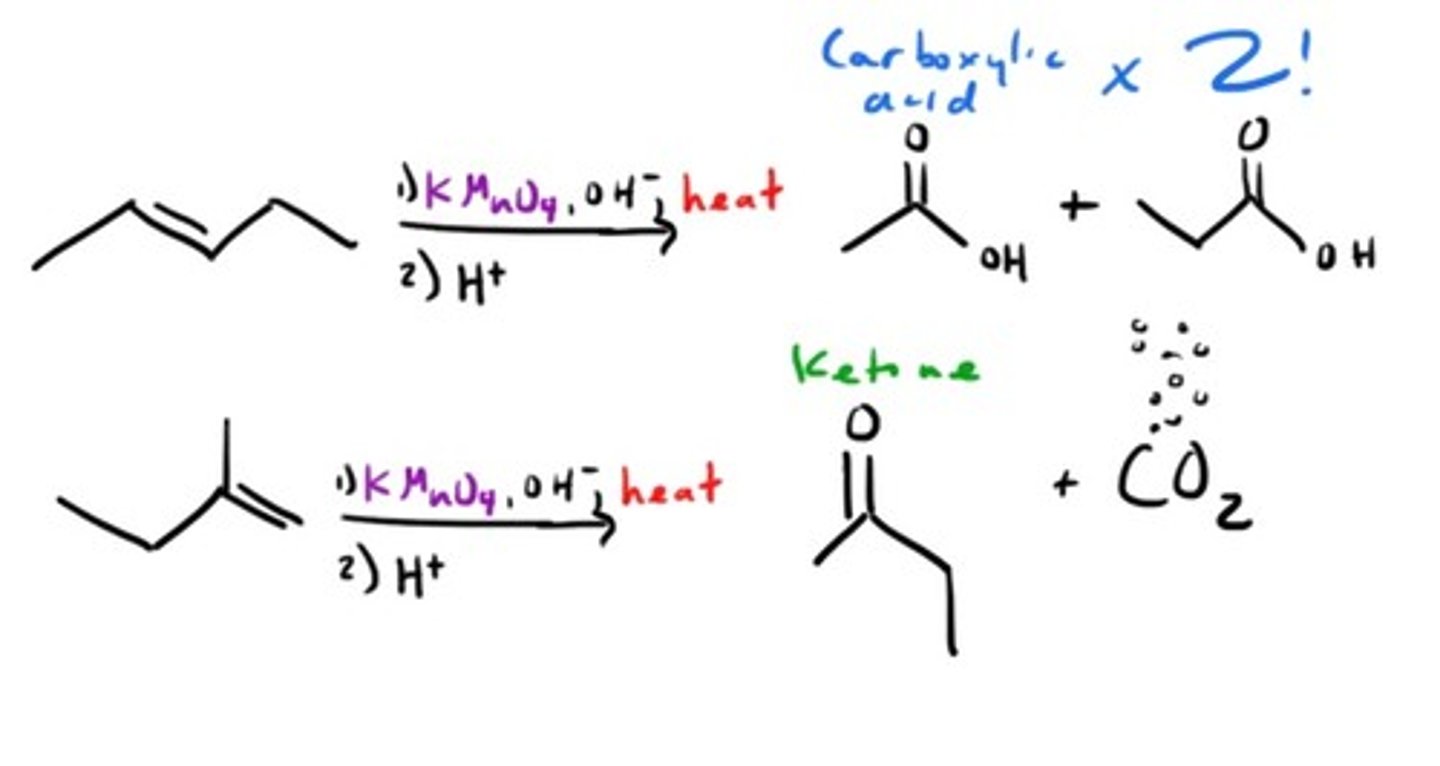
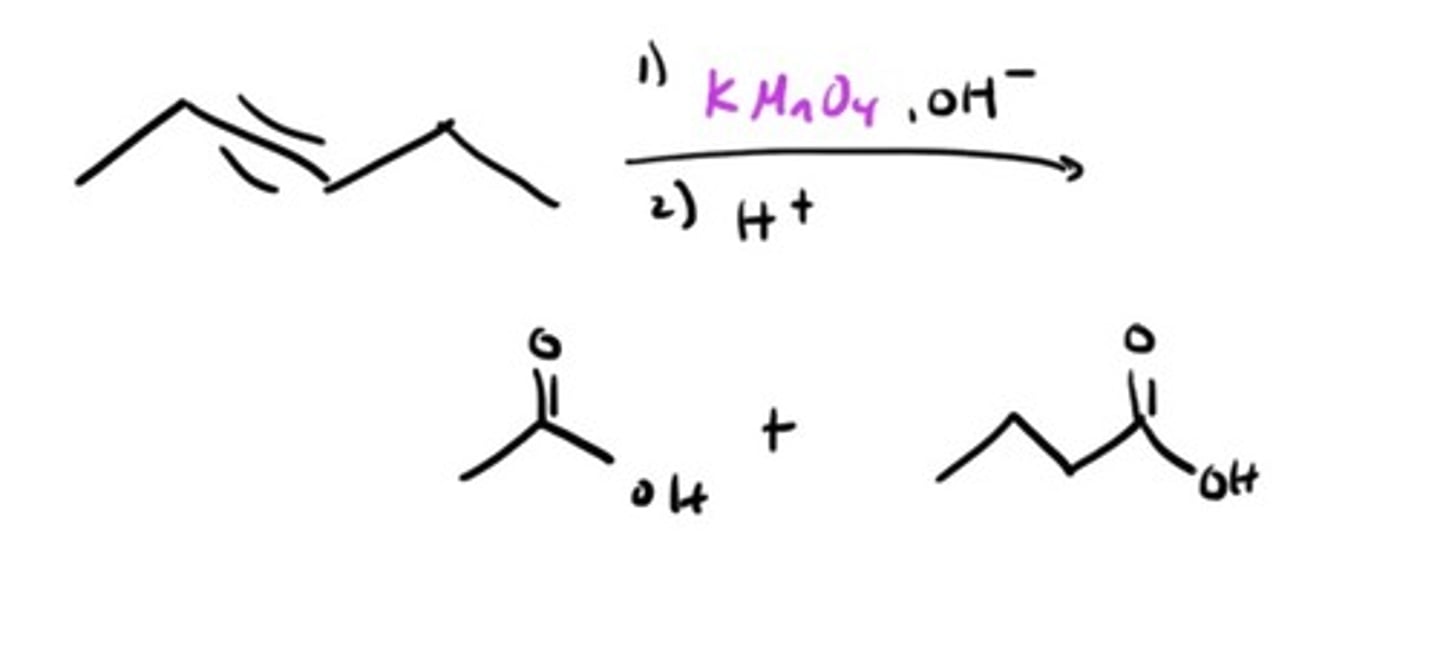
Oxidation with KMnO4 (Hot)
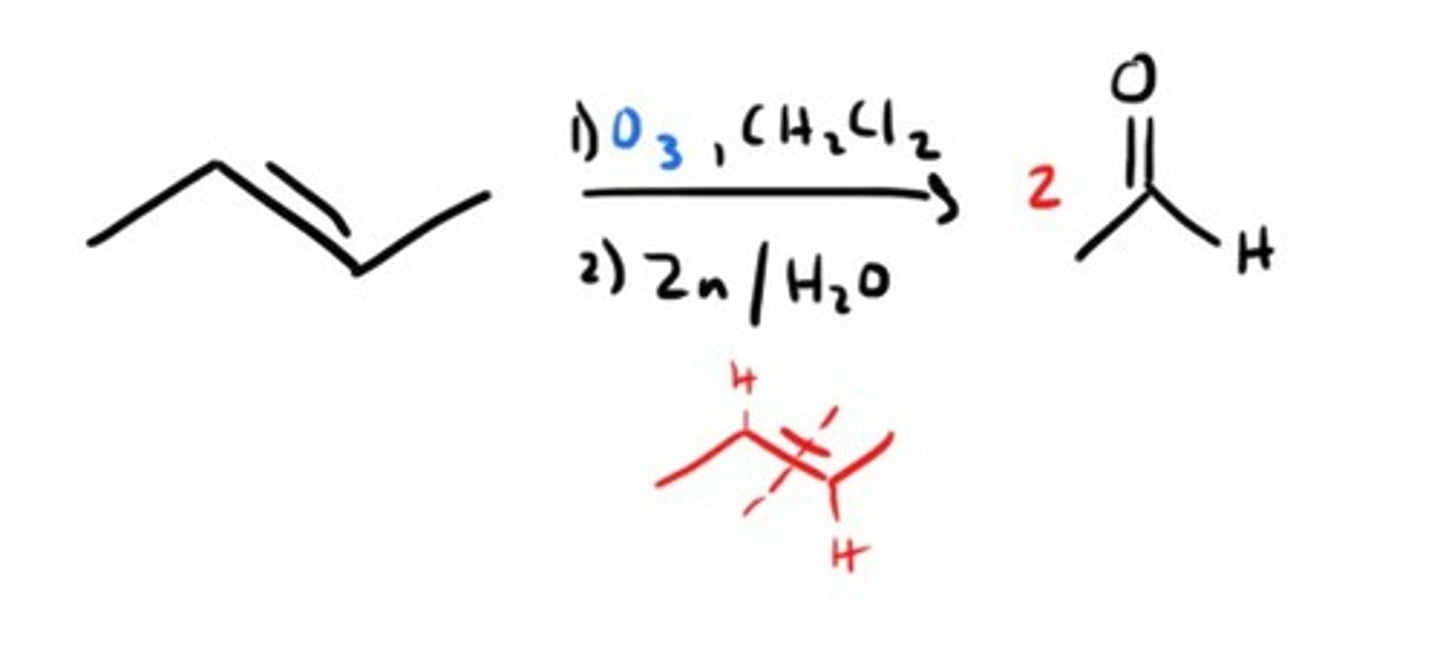
Ozonolysis
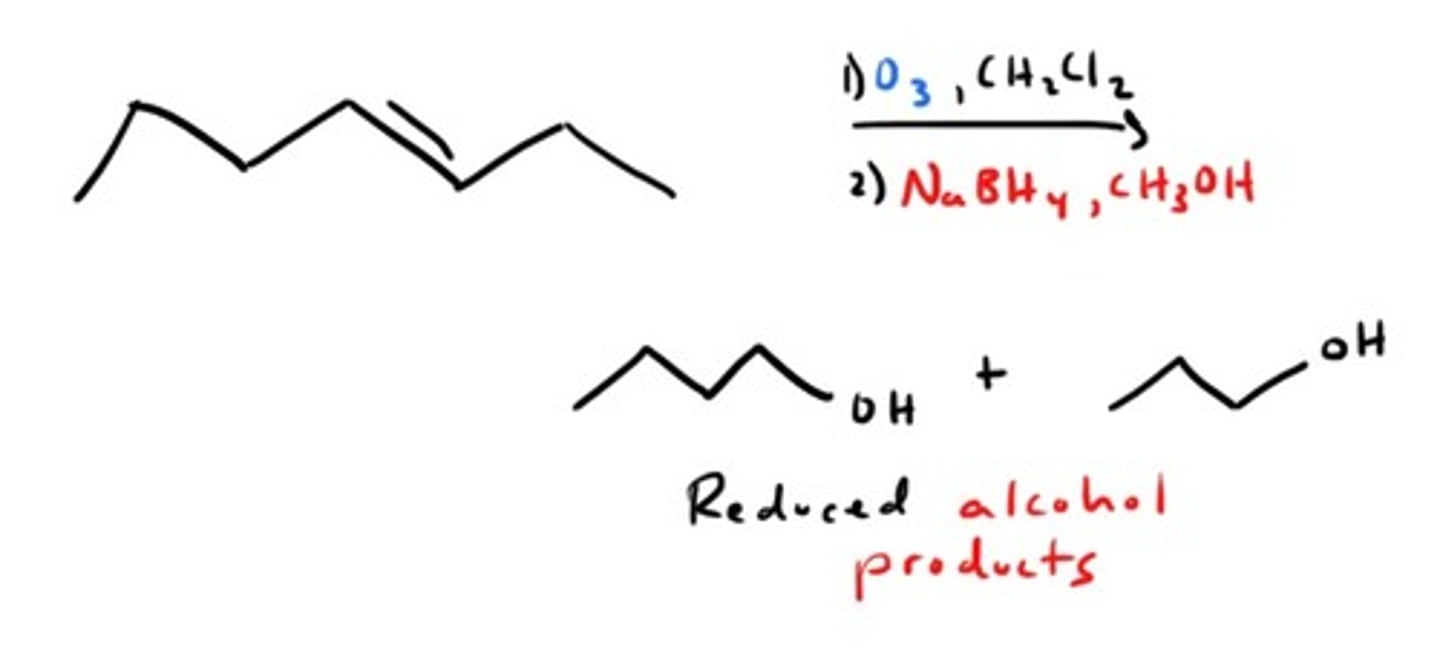
Ozonolysis with Reduction
Peroxycarboxylic acids
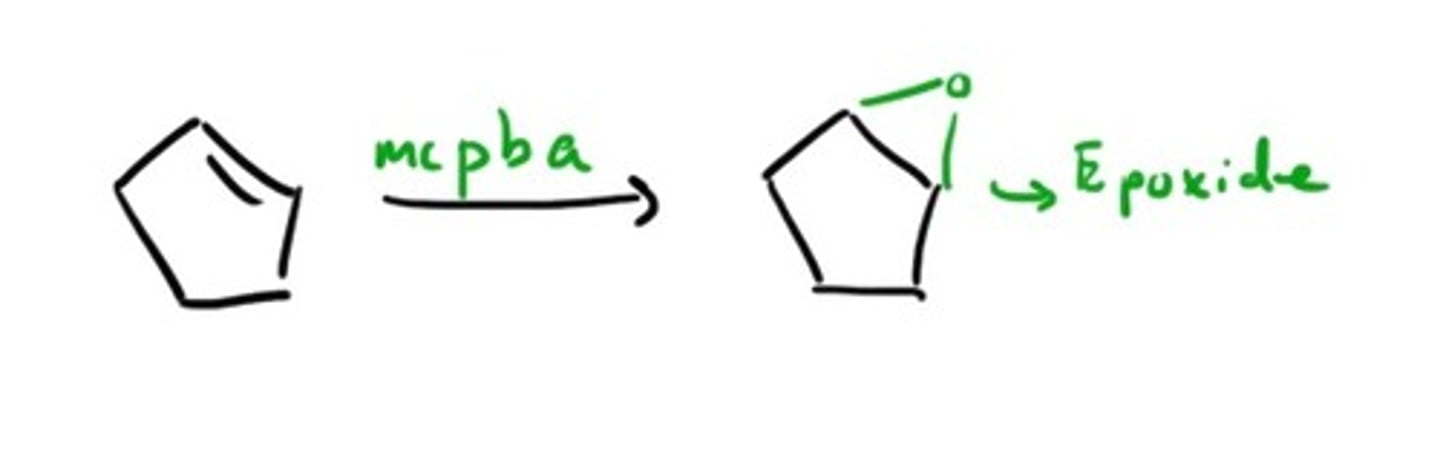
What do you use to add to polymers?
Whats another word for ethyne?
acetylene
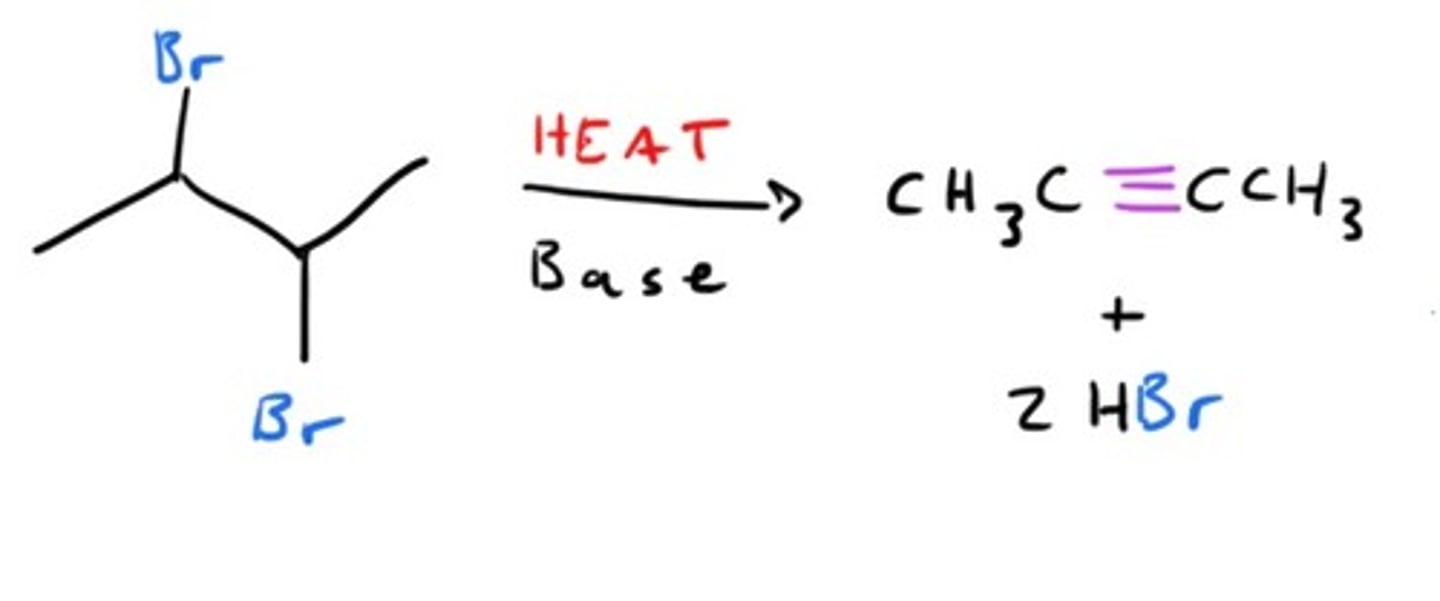
Synthesis of Alkyne starting from dihalide
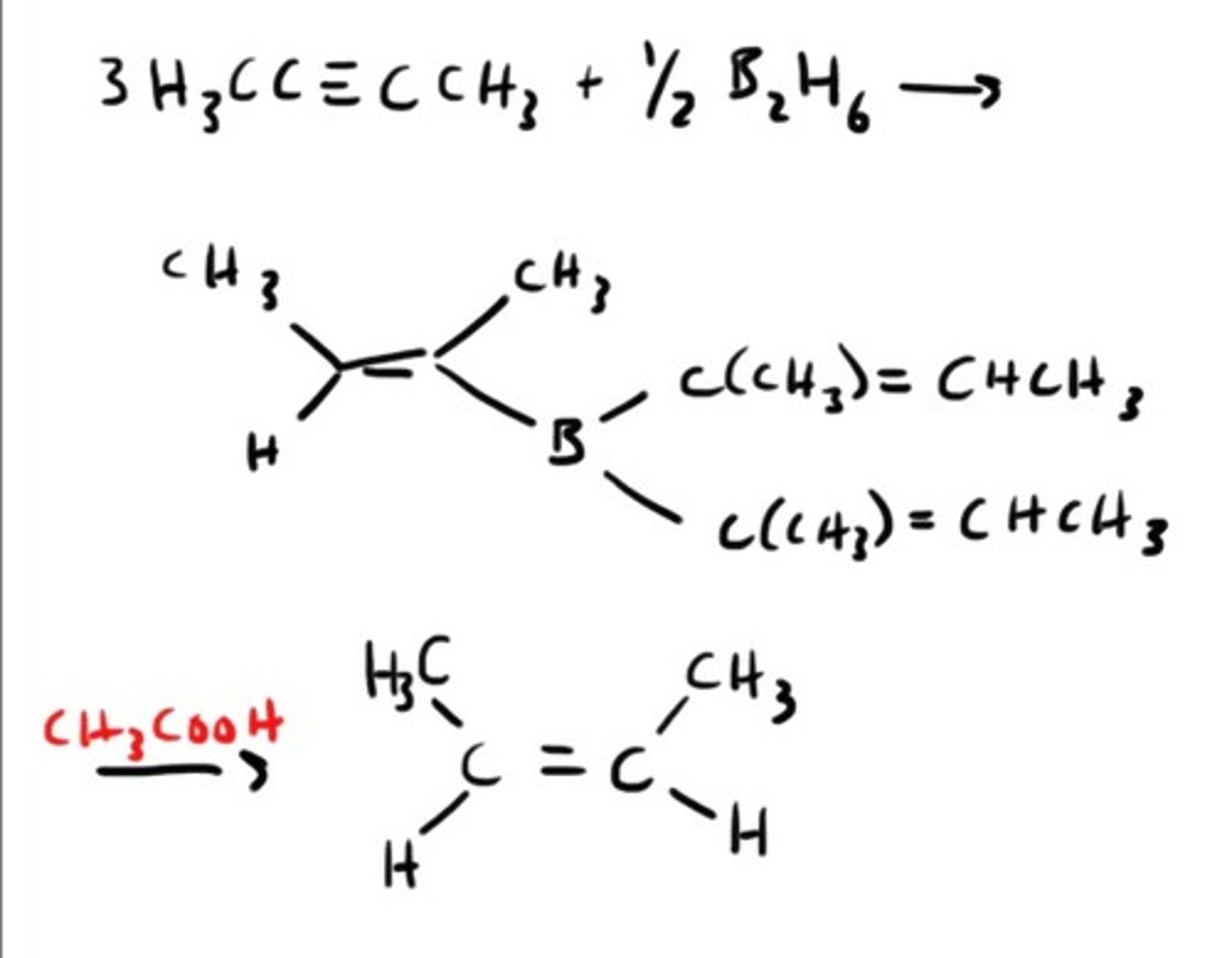
Synthesis of Alkyne using acytelide ion
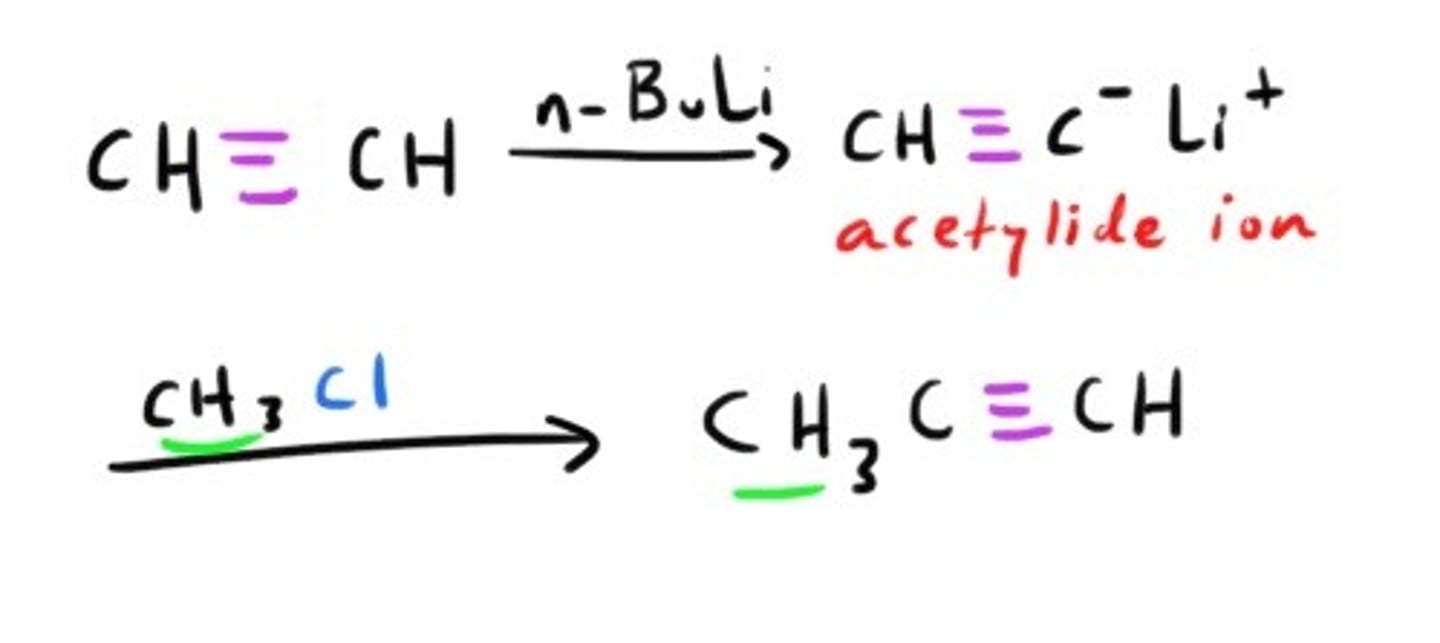
Alkyne Reduction
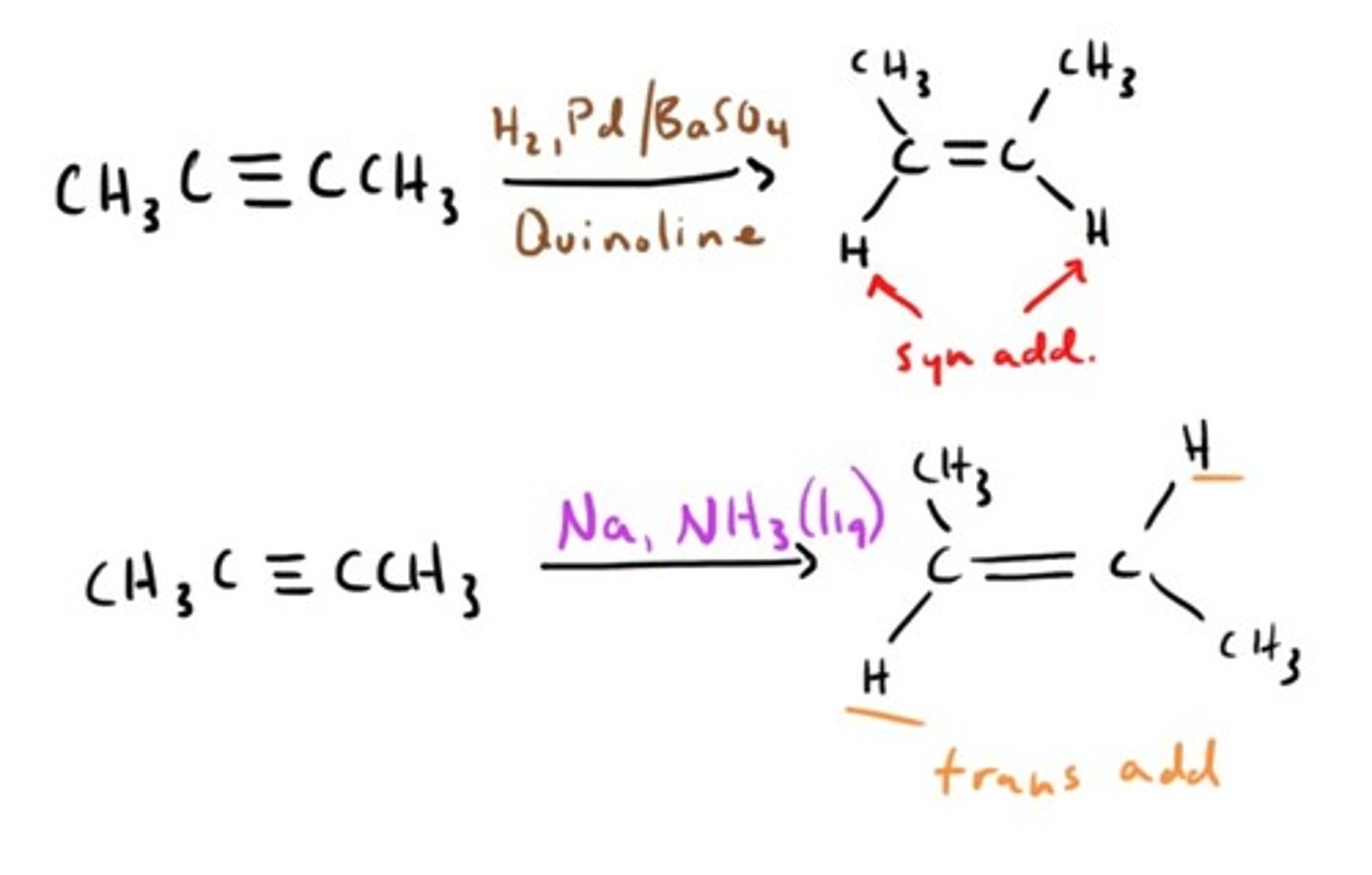
Alkyne Reduction by free radical
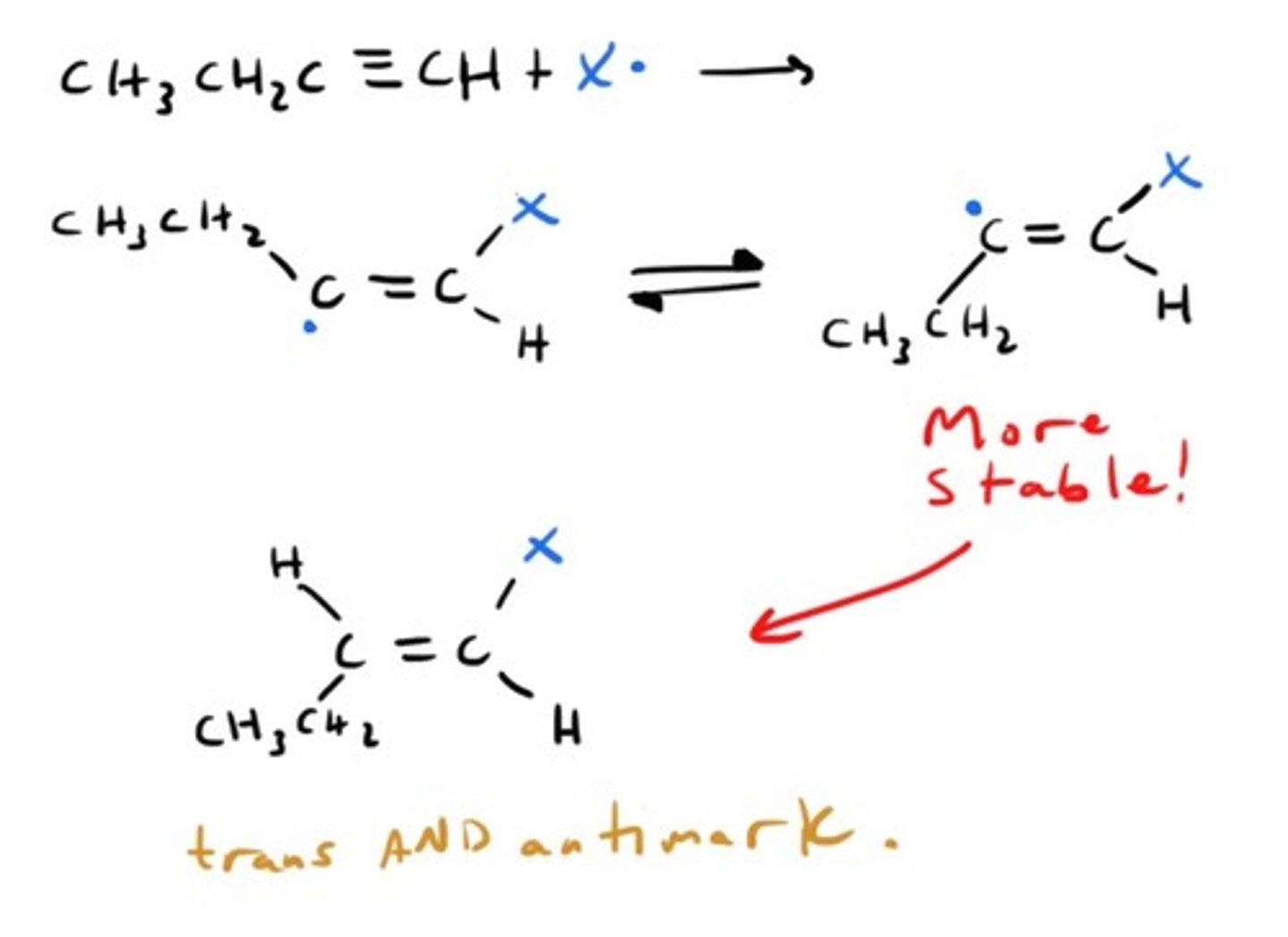
Oxidation of Alkyne by KMnO4
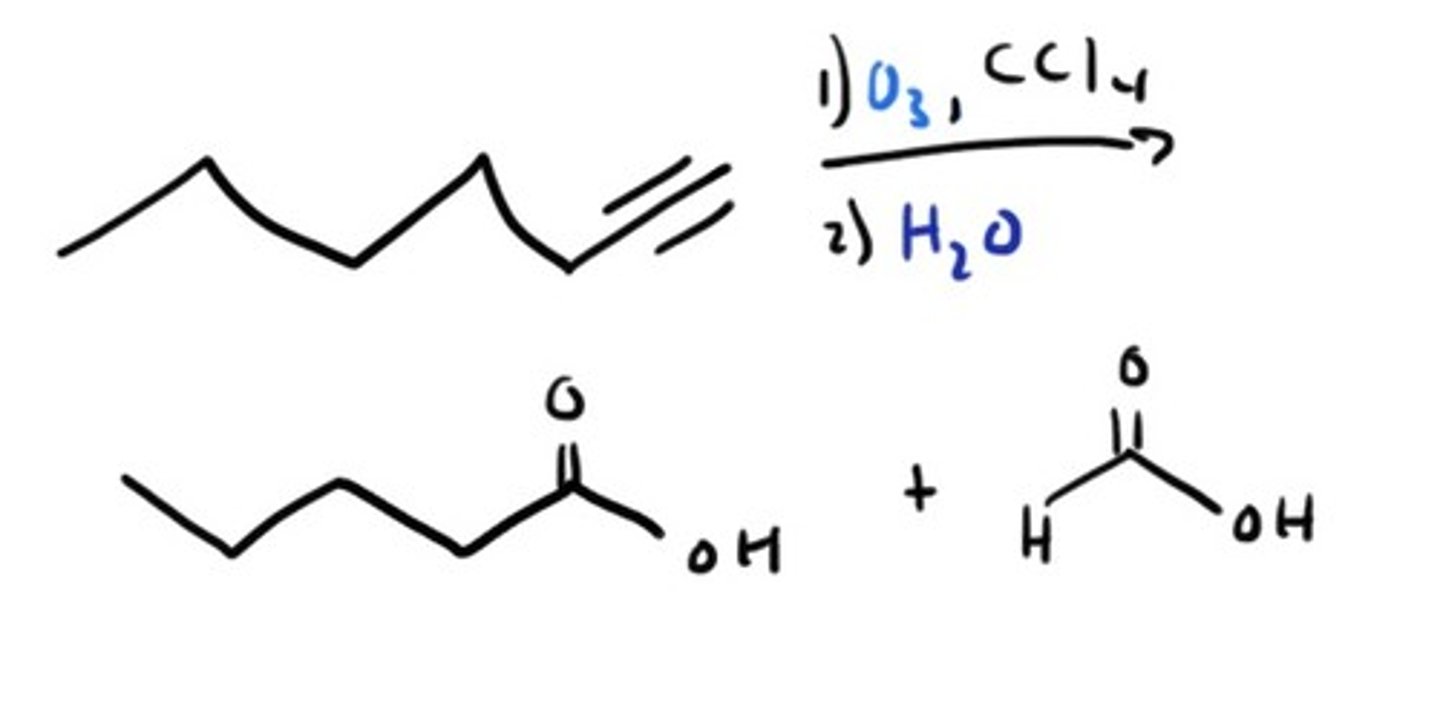
Ozonolysis of Alkyne
Hydroboration of Alkyne
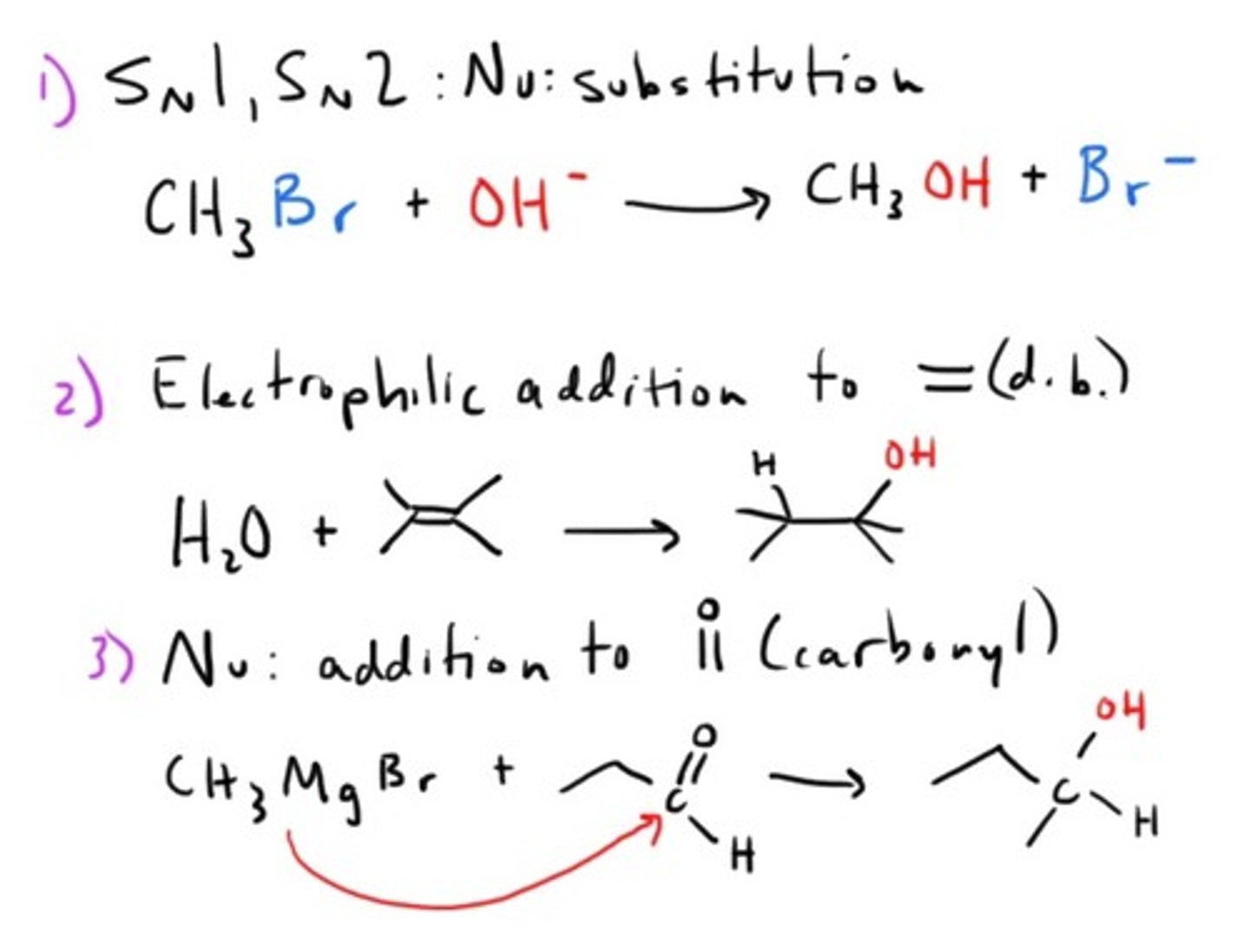
Key reaction mechanisms for Alcohols and Ethers
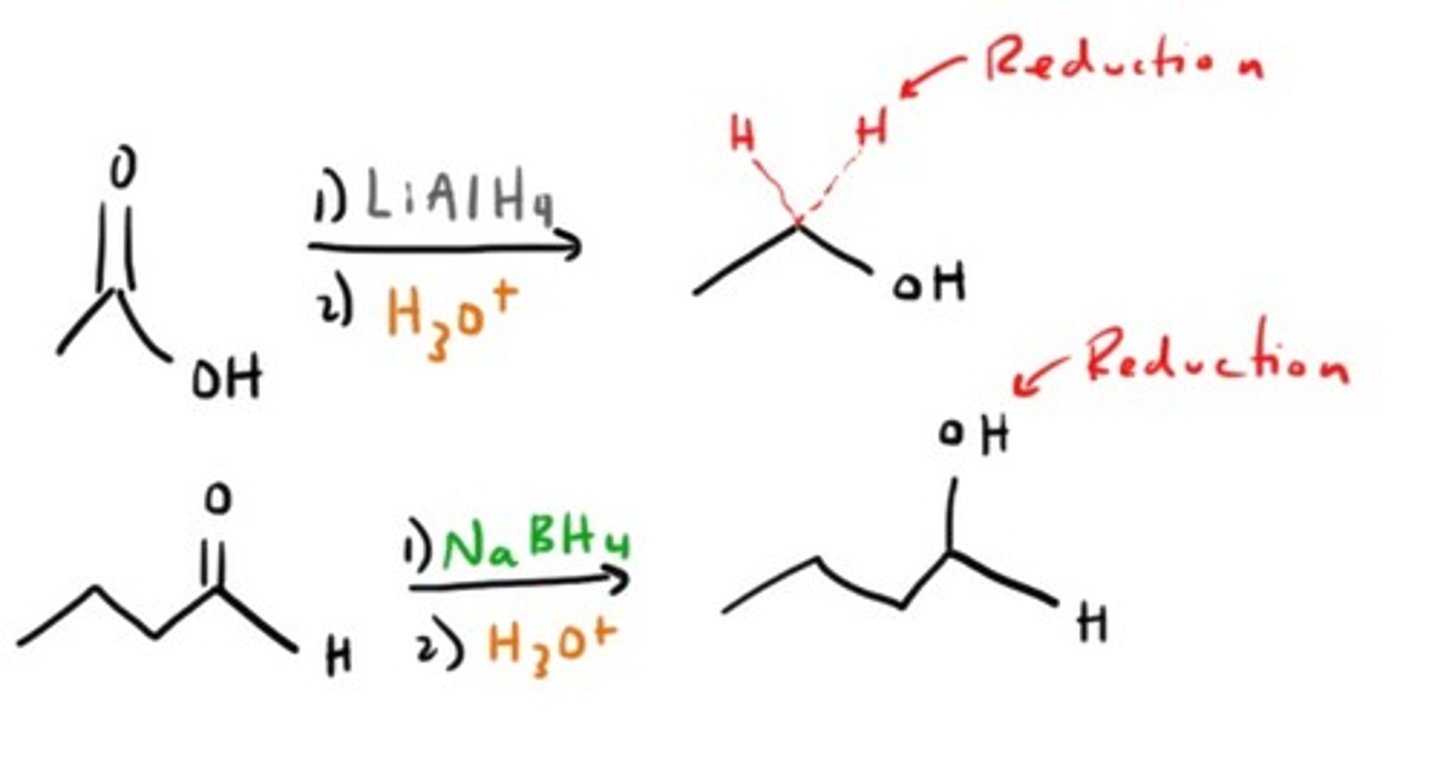
Reduction to make Alcohol
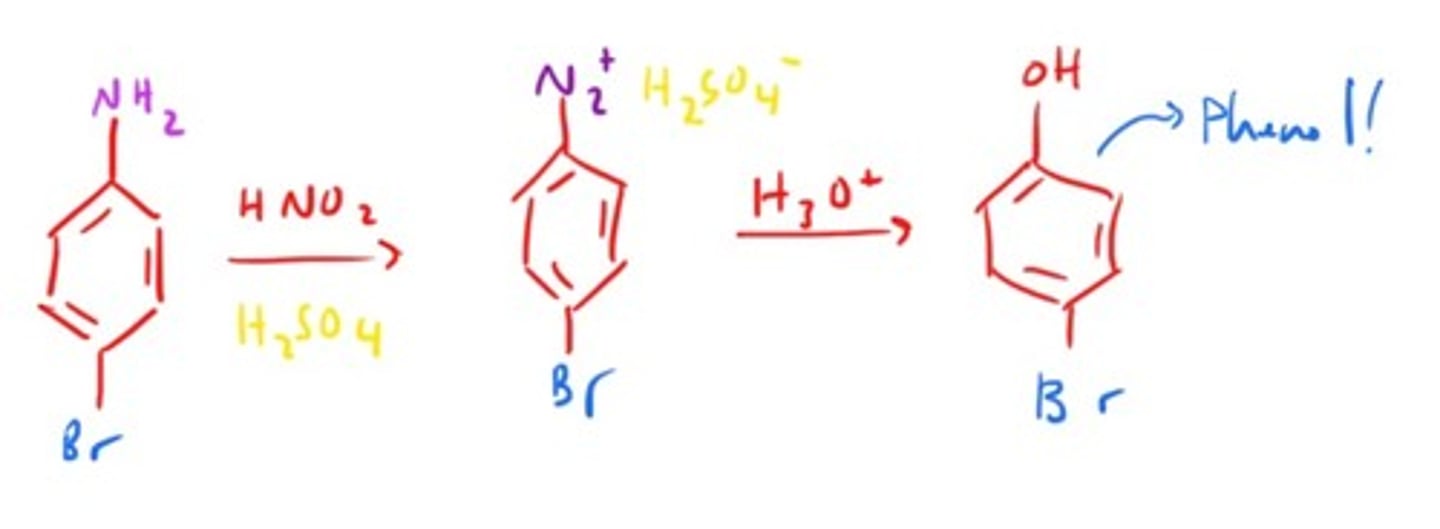
Phenol Synthesis
Alcohol dehydration (elimination)
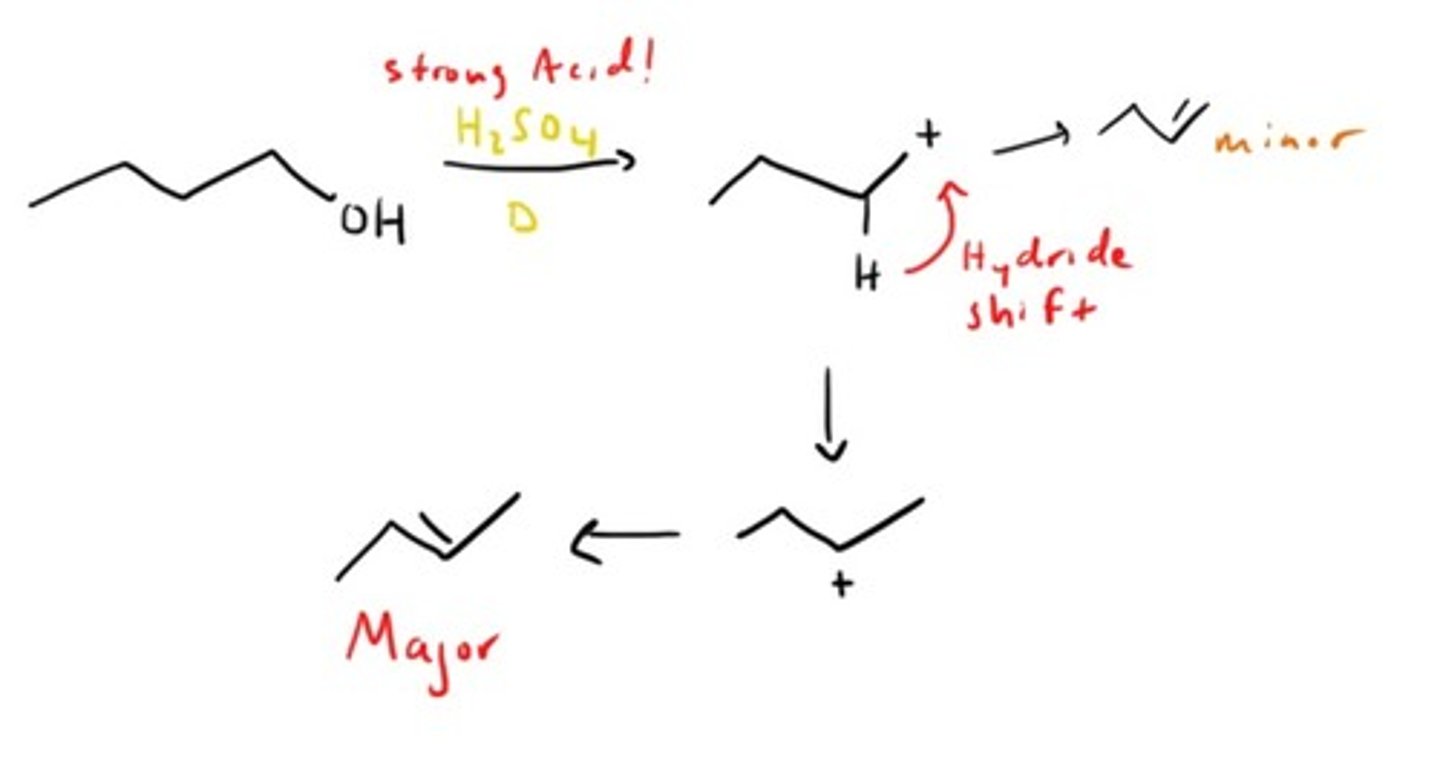
How do you make OH- a good leaving group for Sn1 and Sn2 reactions?
For Sn1 you can protonate it and make water the leaving group.
For Sn2 you can convert it to tosylate which is also a good leaving group.
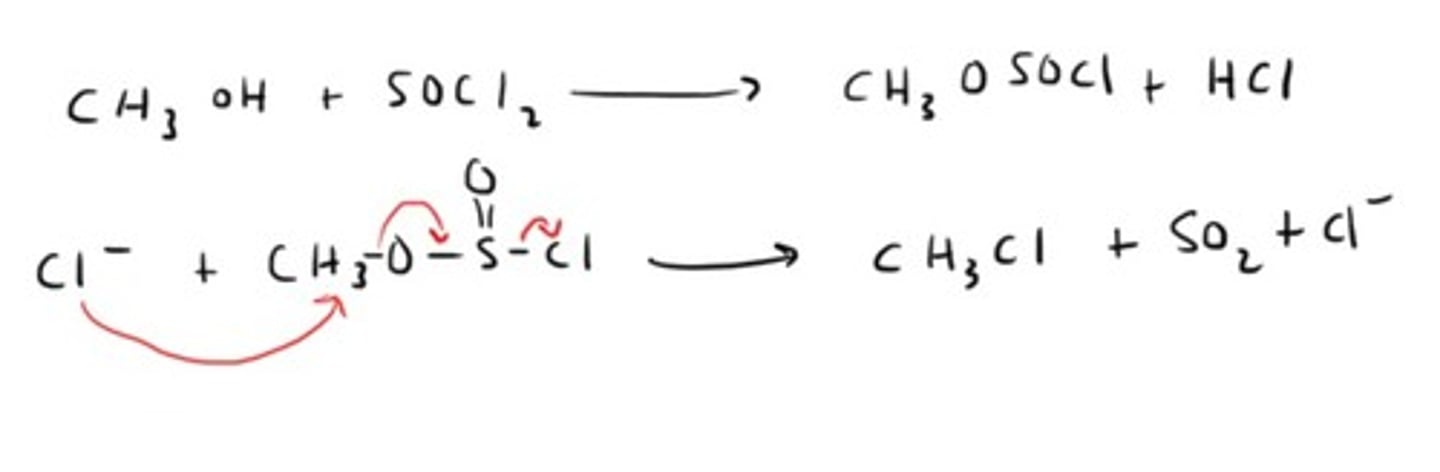
How do you convert an alcohol into an alkyl halide with an ester as an intermediate?
What does PCC(pyridinium chlorochromate) do?
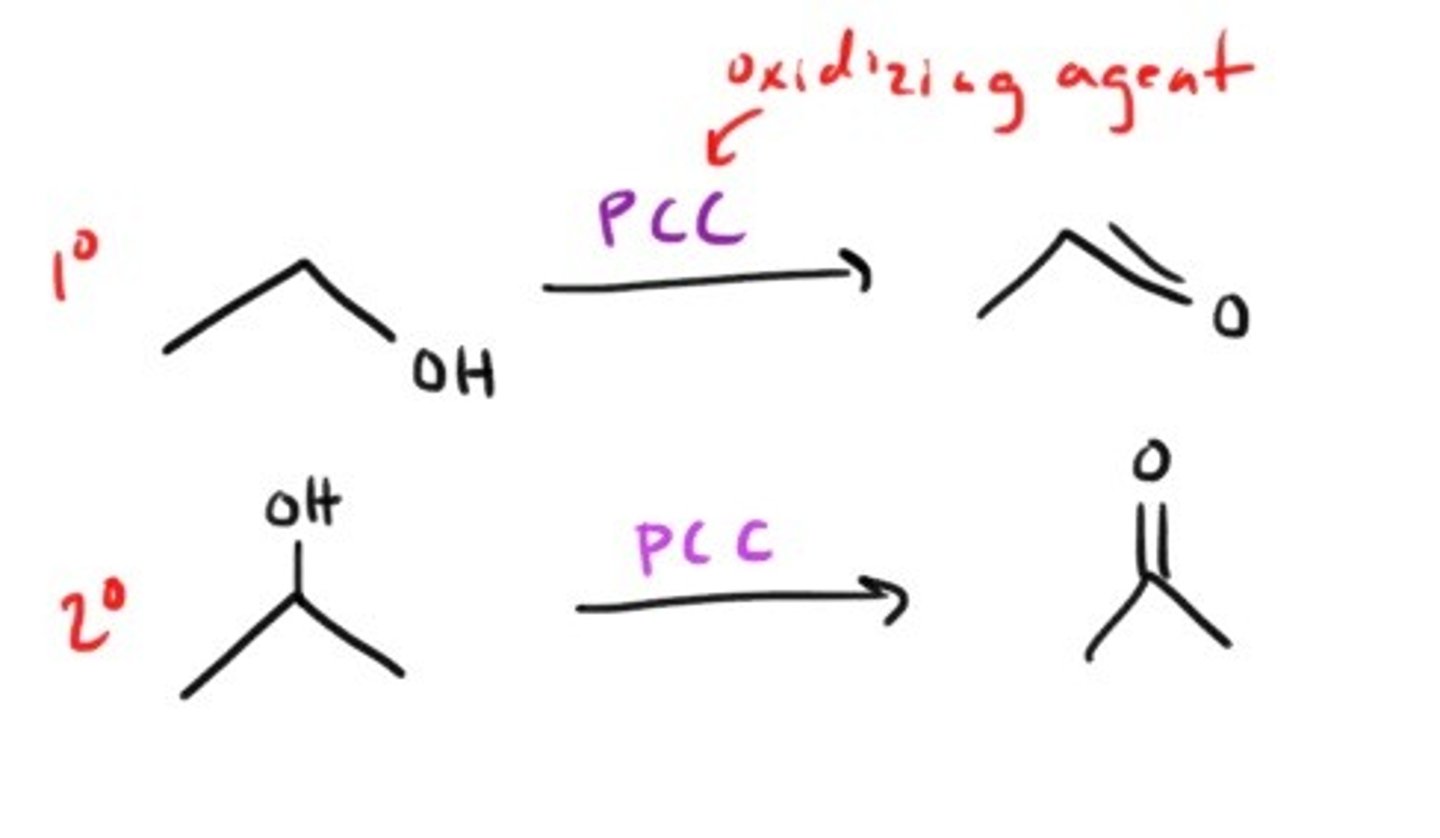
What is jones reagent and what does it do?
it is CrO₃, H₂SO₄ in acetone and it oxidizes primary alcohols to carboxylic acids (STRONG!)
Do ethers boil at high or low temperatures?
Low...no H-bonding.
William Ether Synthesis
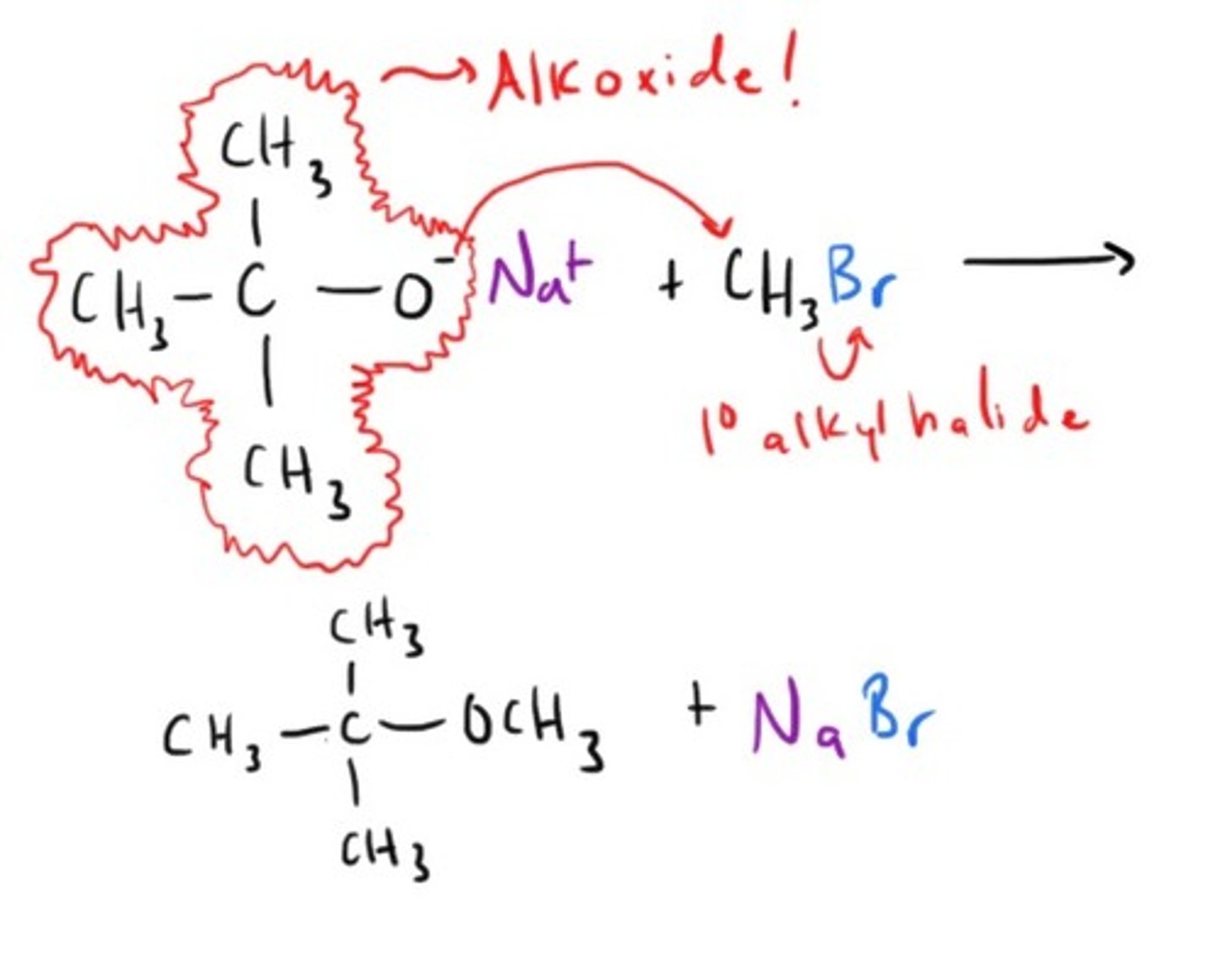
How to Cleave an Ether!
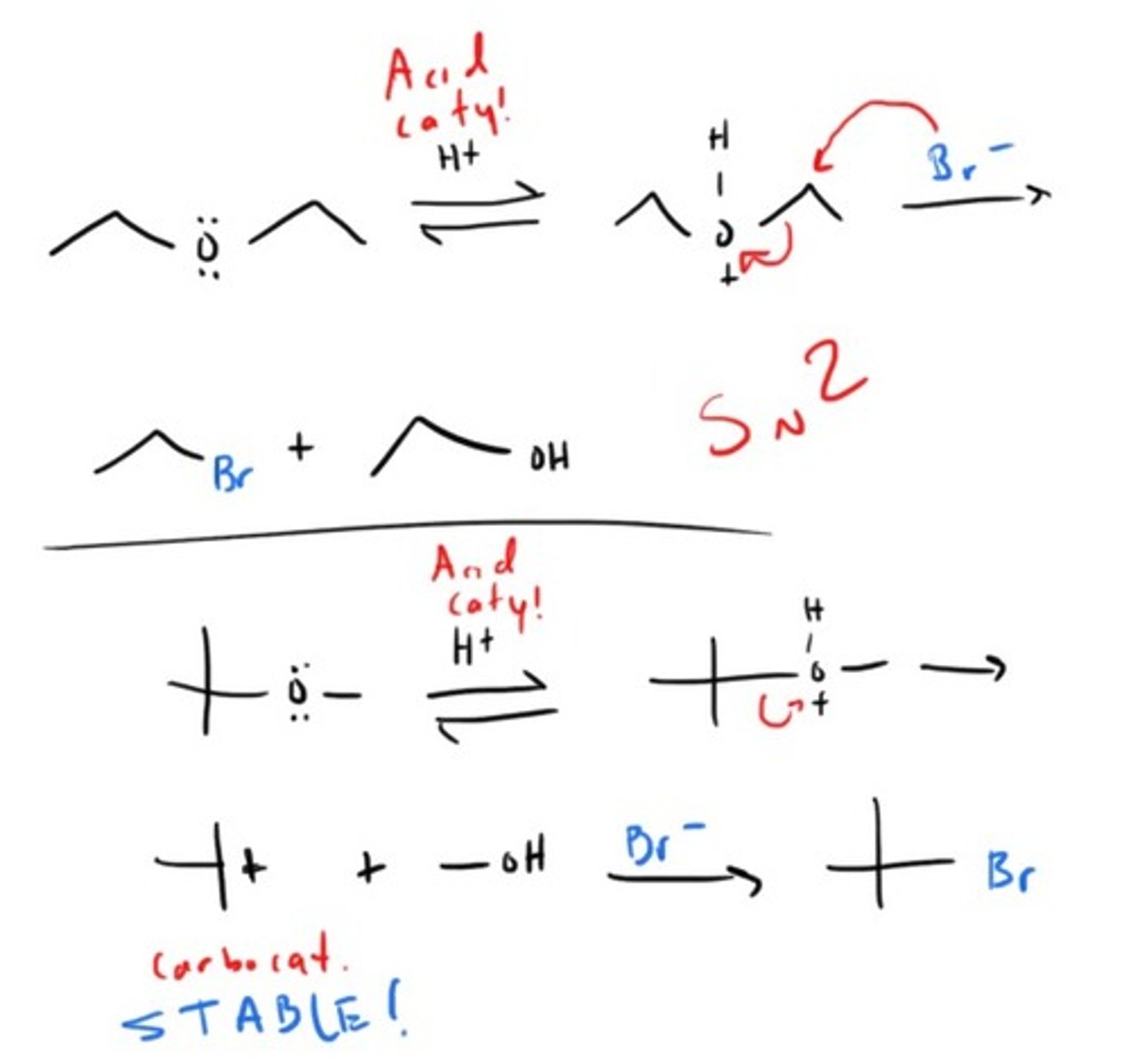
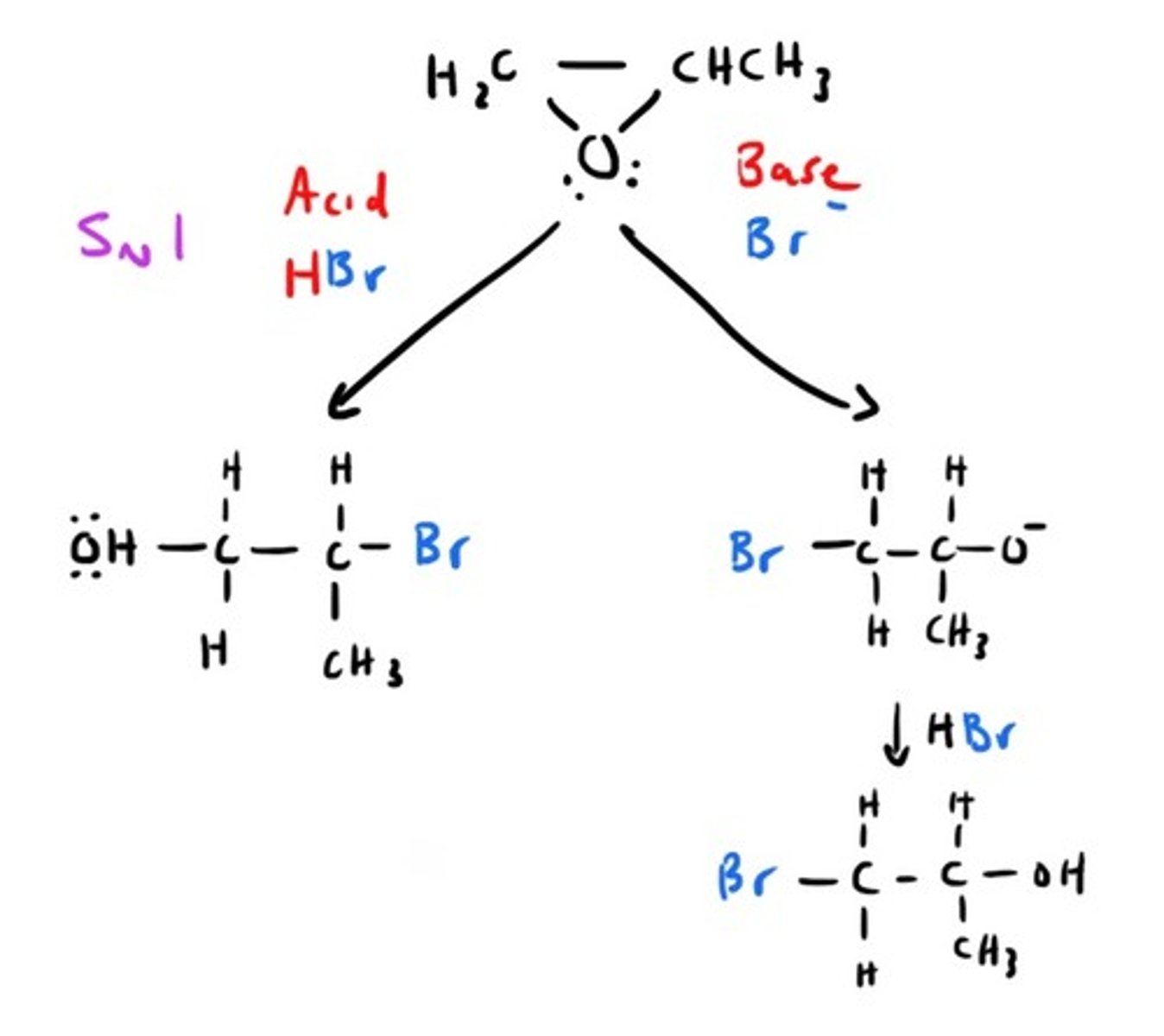
How can we go from Epoxide to Alcohol?
How can you get ketones or aldehydes?
Oxidation of primary or secondary alcohols. Or ozonolysis of alkenes.
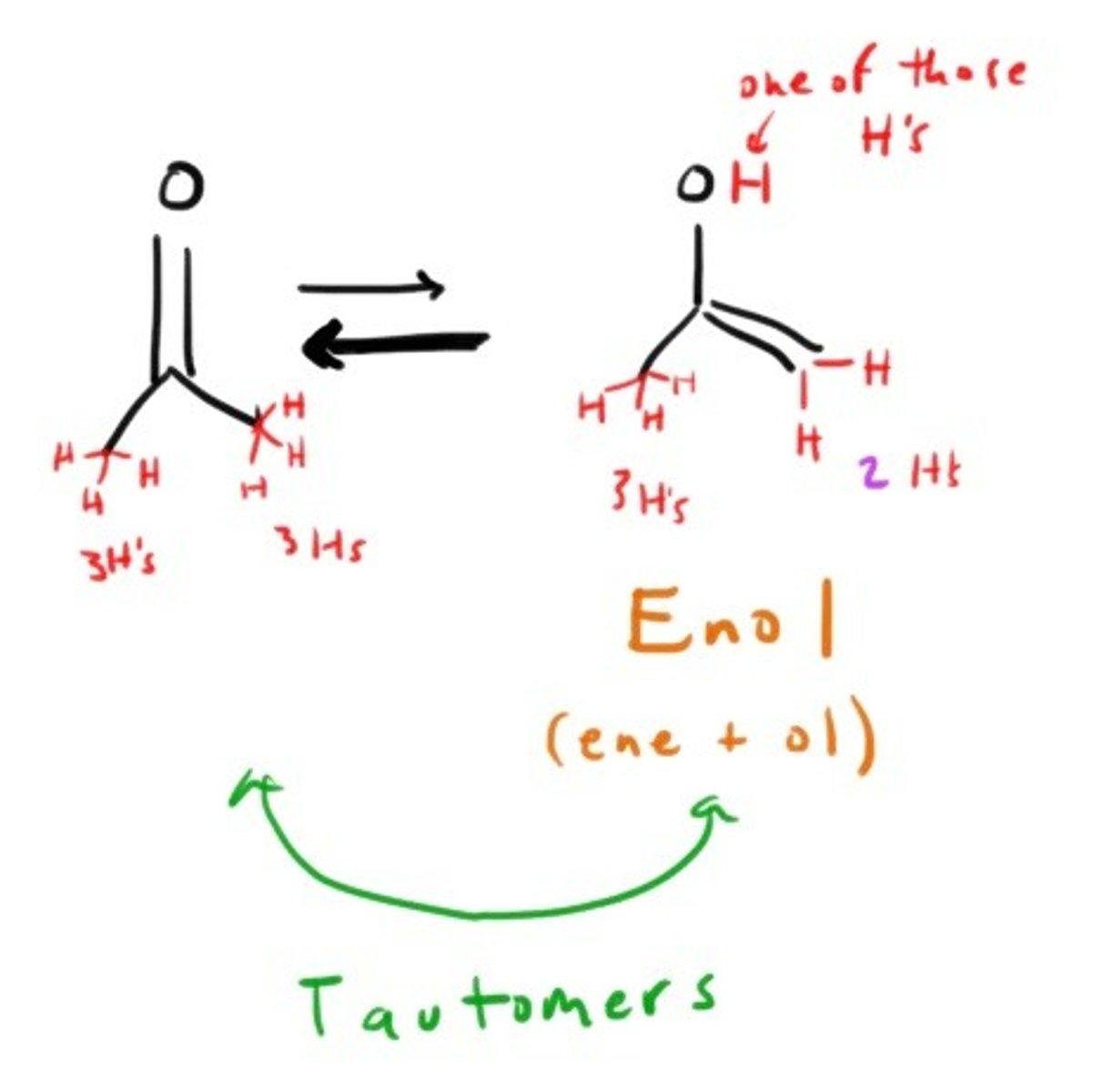
What happens between Ketones and Enols?
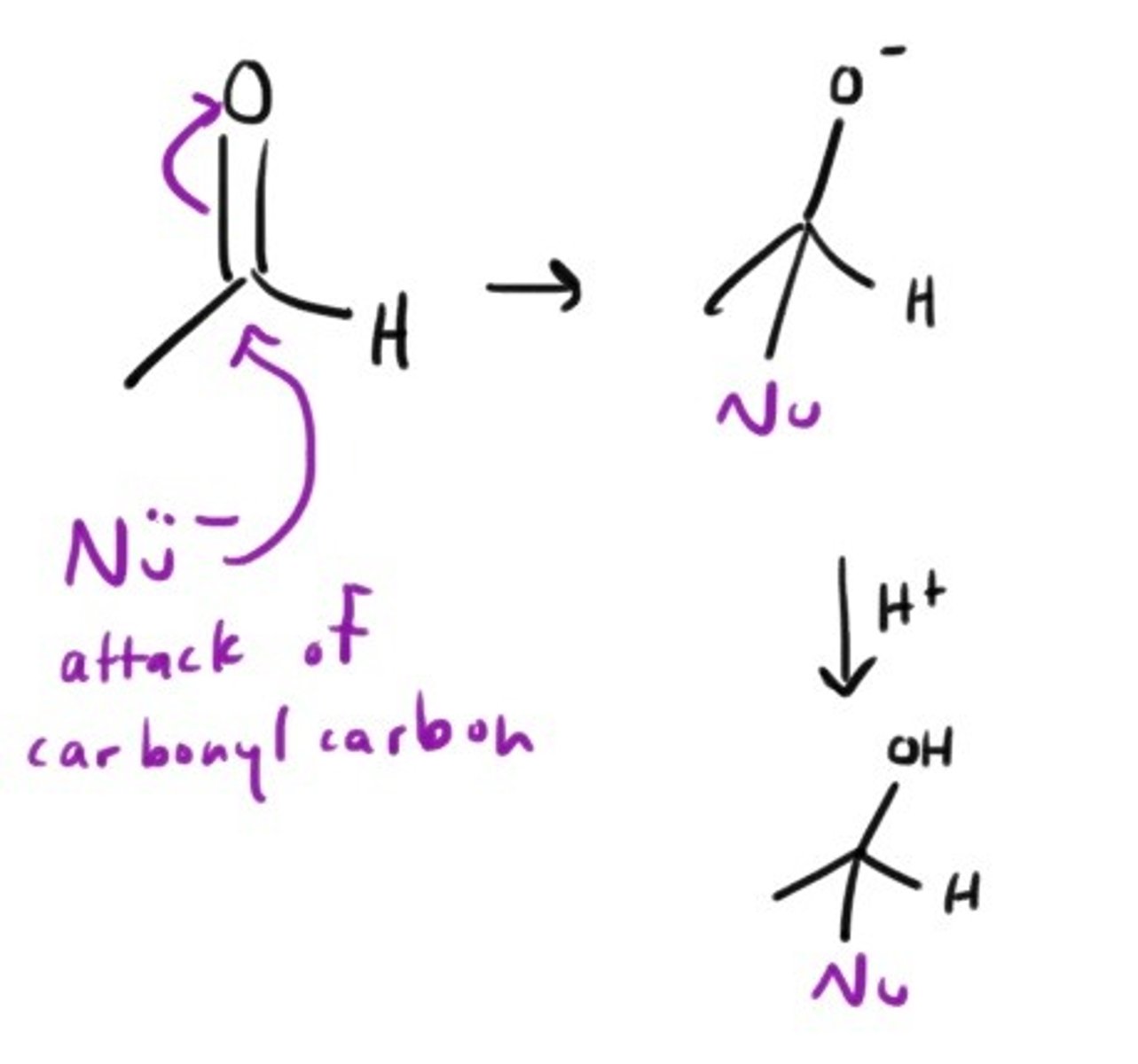
Basic attack of Nu on Carbonyl Carbon.
How do you get an acetal or ketal?
When an aldehyde or ketone reacts with two equivalents of alcohol. A hemiketal or hemiacetal you get from one equivalent of alcohol.
What is a cyanohydrin?
When al and ke react with HCN.
Ammonia leading to condensation...
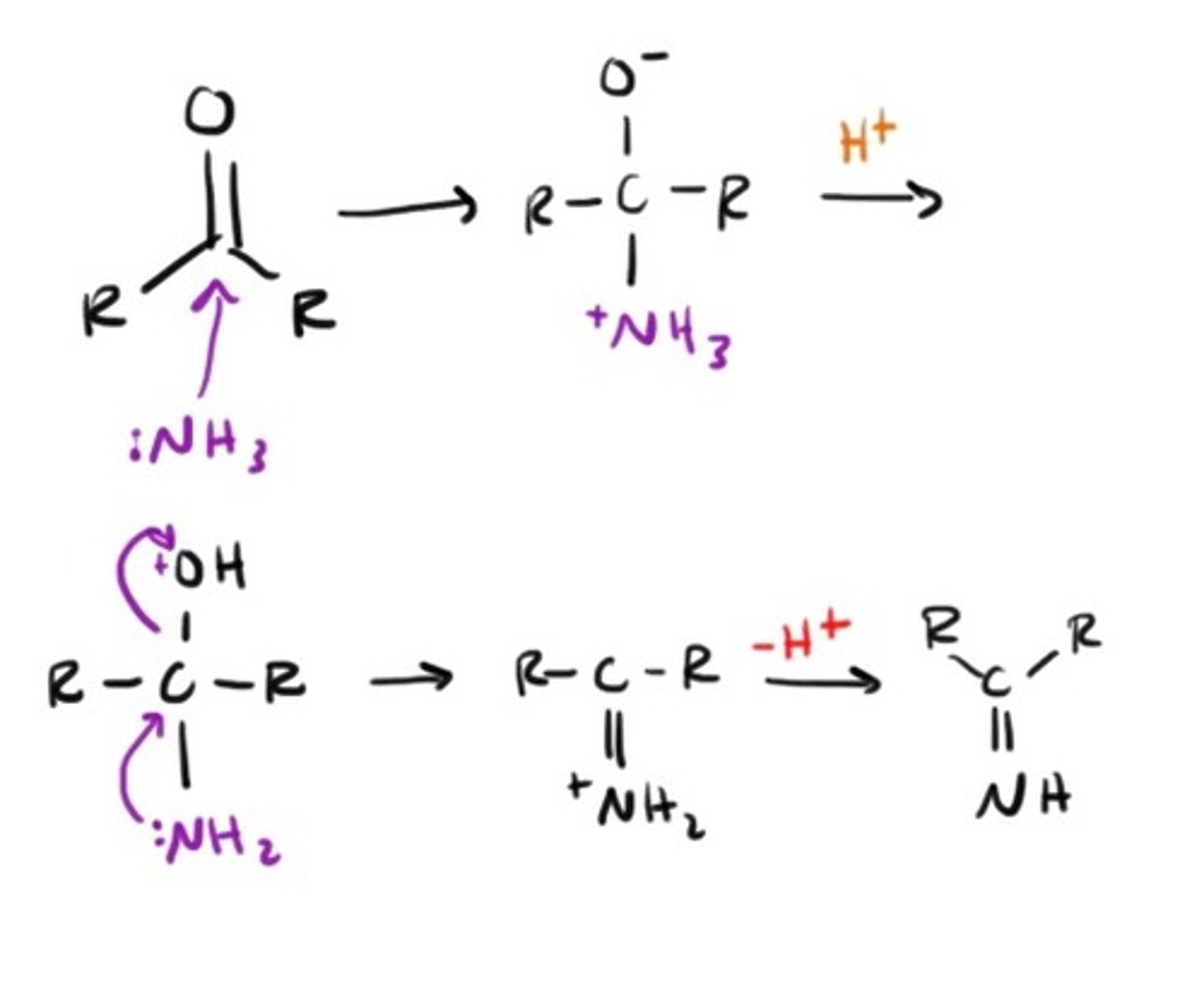
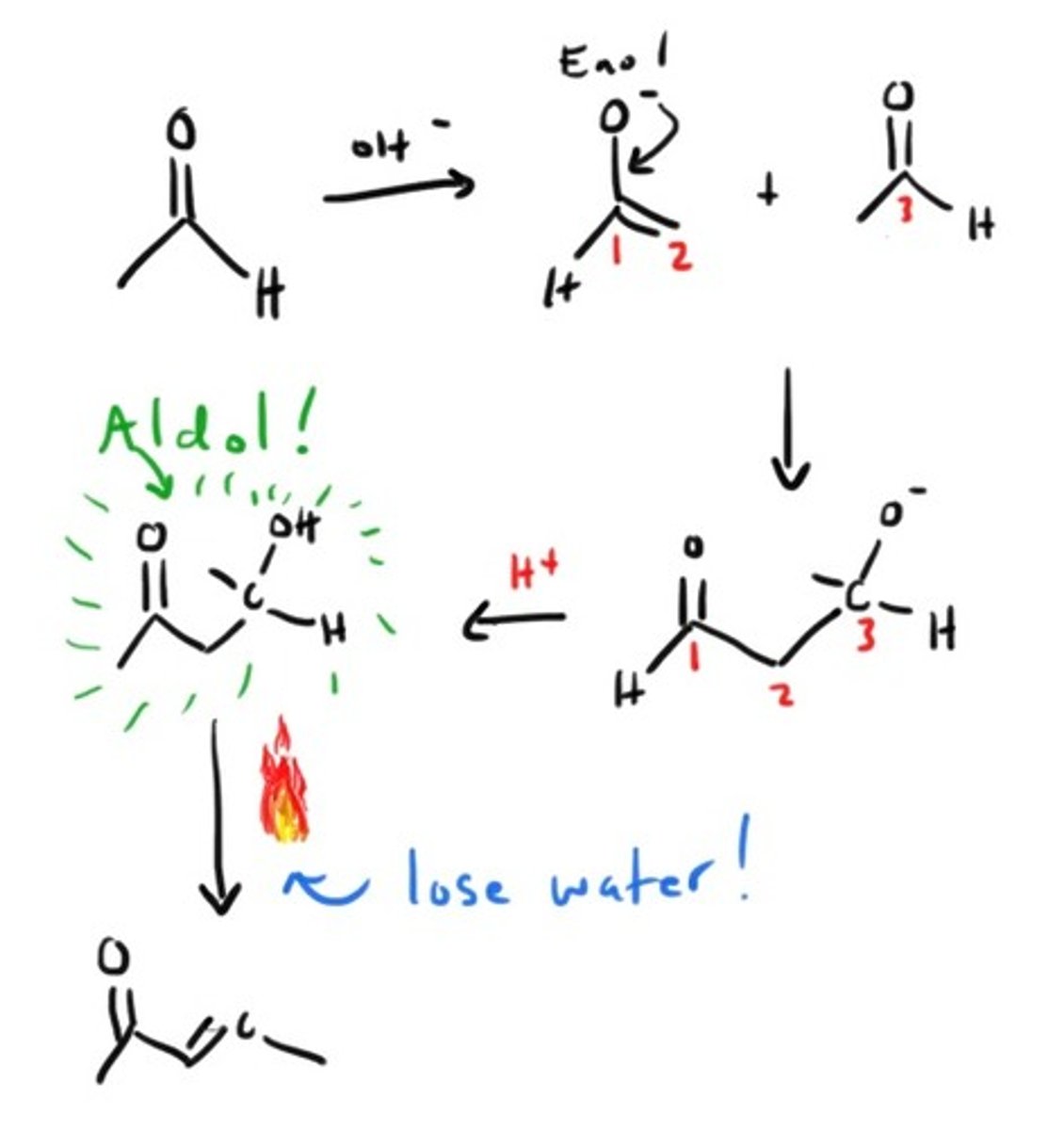
Aldol Condensation
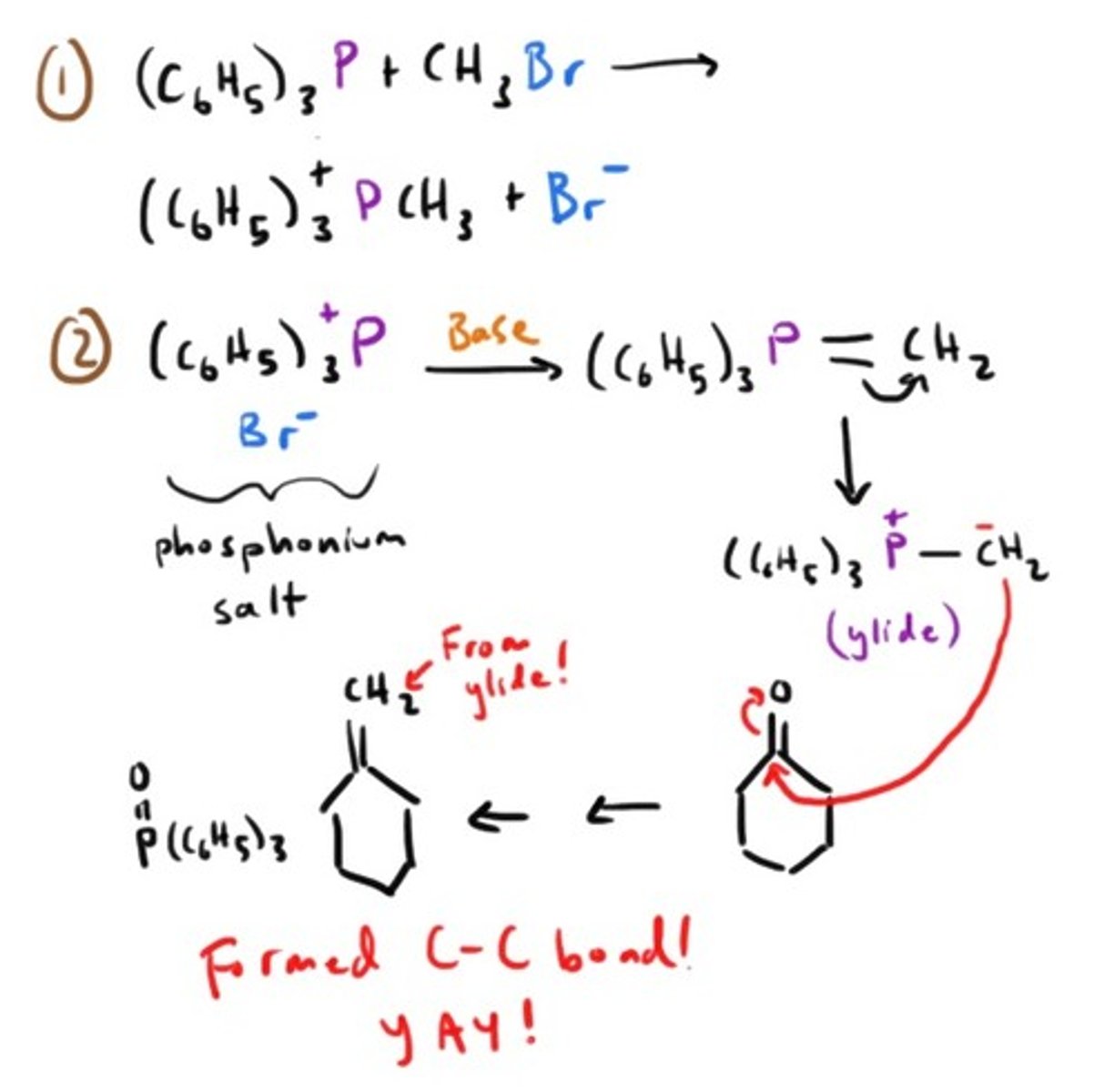
What is a wittig reaction and what is its goal?
The goal is to make CARBON-CARBON double bonds by converting aldehydes and ketones into alkenes.
What oxidizes an aldehyde and what do you get?
KMnO₄, CrO₃, Ag₂O, H₂O₂ and you get a carboxylic acid.
What reduces aldehydes and ketones?
LAH or NaBH₄
What are the reagents for Wolff-Kishner Reduction of al and ke?
H₂NNH₂, then a base and heat.
What are the reagents for Clemmensen Reduction?
Hg(Zn) and HCl
Why are carboxylic acids that much more acidic?
Resonance stabilization of between the alcoholic oxygen and the carbonyl oxygen.
Electron withdrawing vs donating...what do each do to a negative charge and acidity?
Withdrawing delocalize/stabillize the negative charge (spread it over the molecule) which increase acidity. Donating do the opposite.
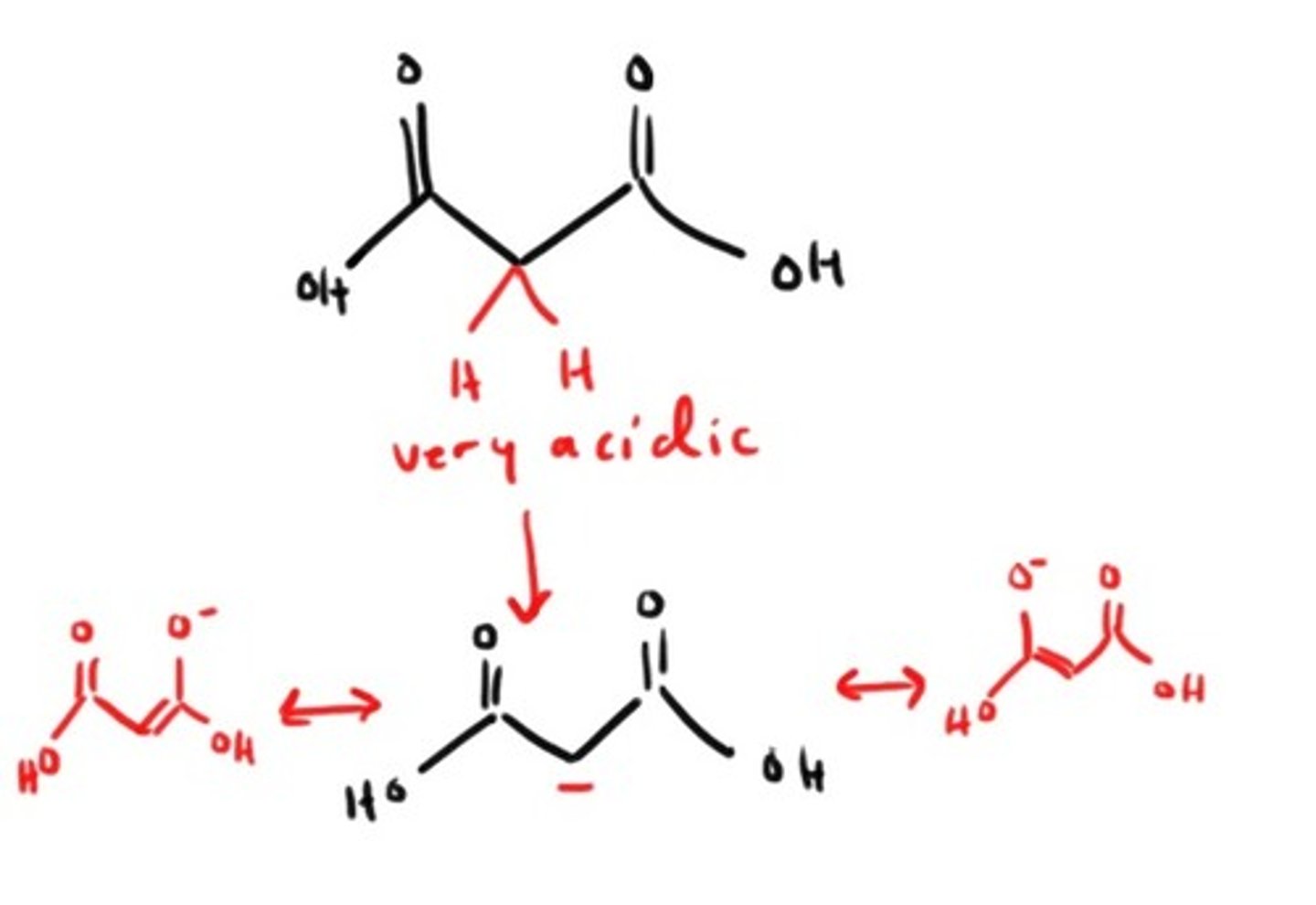
What is significant about the α hydrogens on a β-dicarboxylic acid?
They are very acidic due to stabalization!
Only primary alcohols can be oxidized to carboxylic acids. T/F?
True!
How is carbonation of an orgmet reagent achieved?
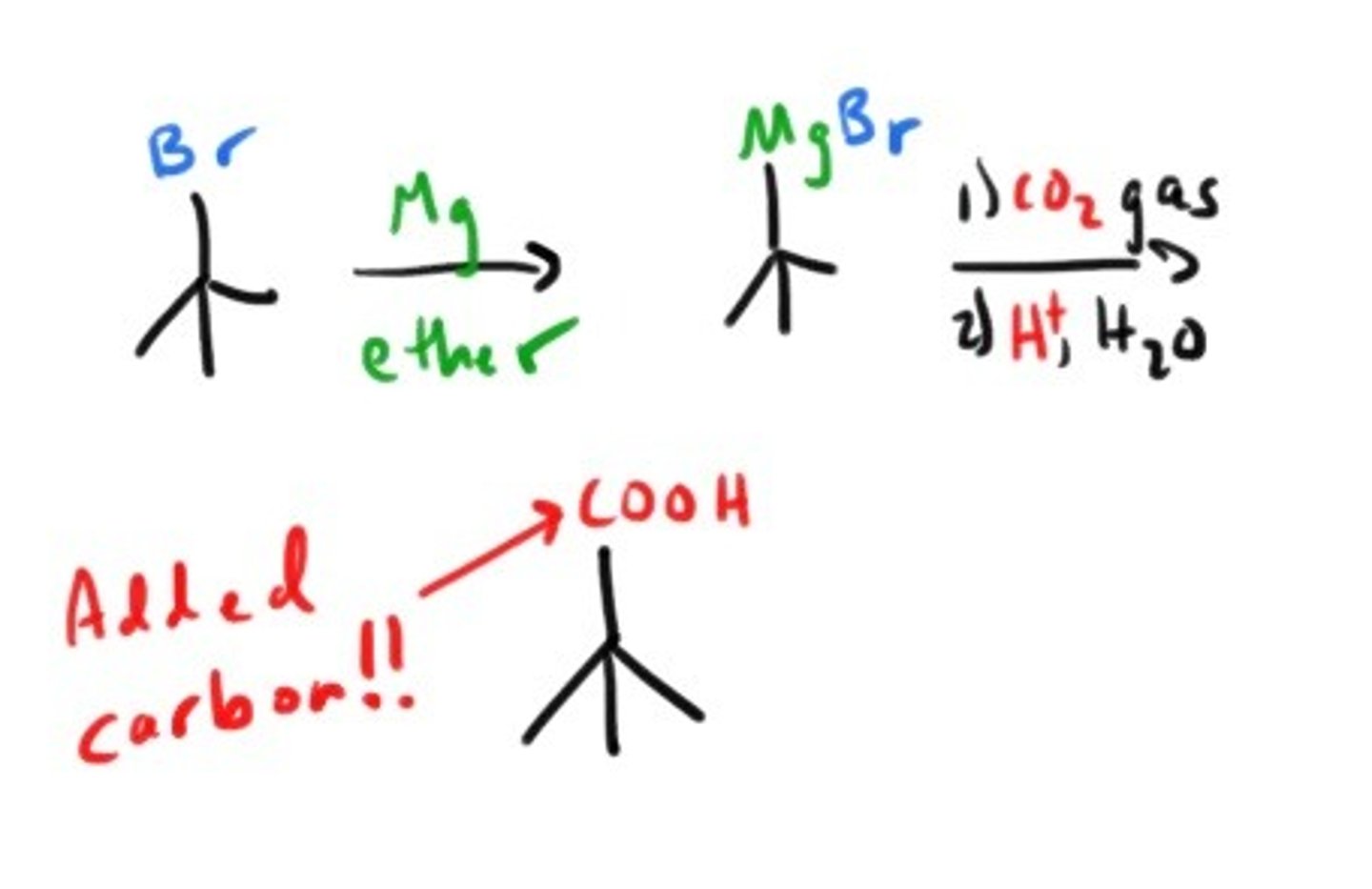
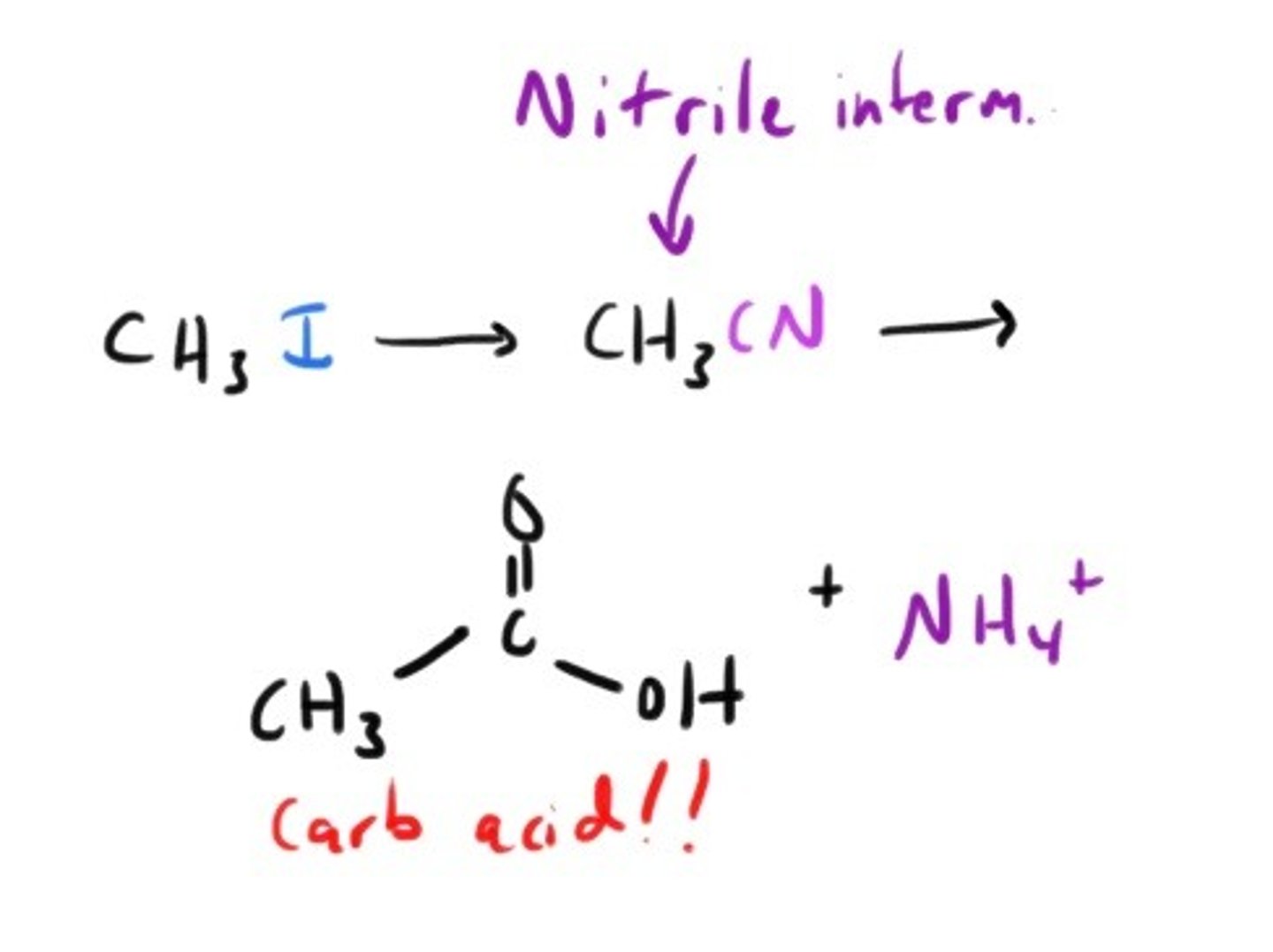
Is there a way to convert primary and secondary alkyl halides into carboxylic acids? Sure!!
nitrile formation followed by acid or base catalyzed hydrolysis.
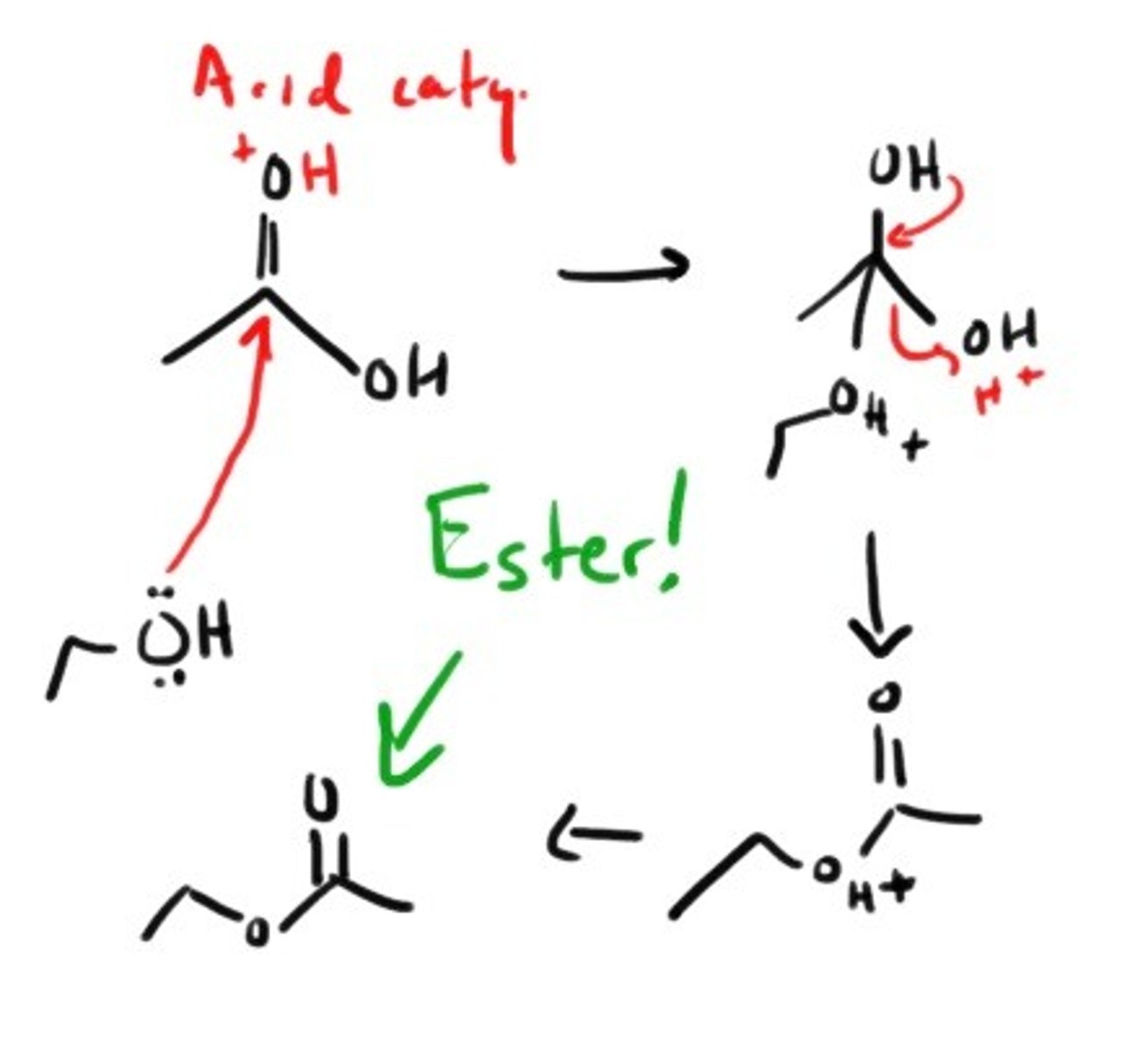
How do you form an ester with a carboxylic acid?
Addition of a primary alcohol under ACIDIC conditions.
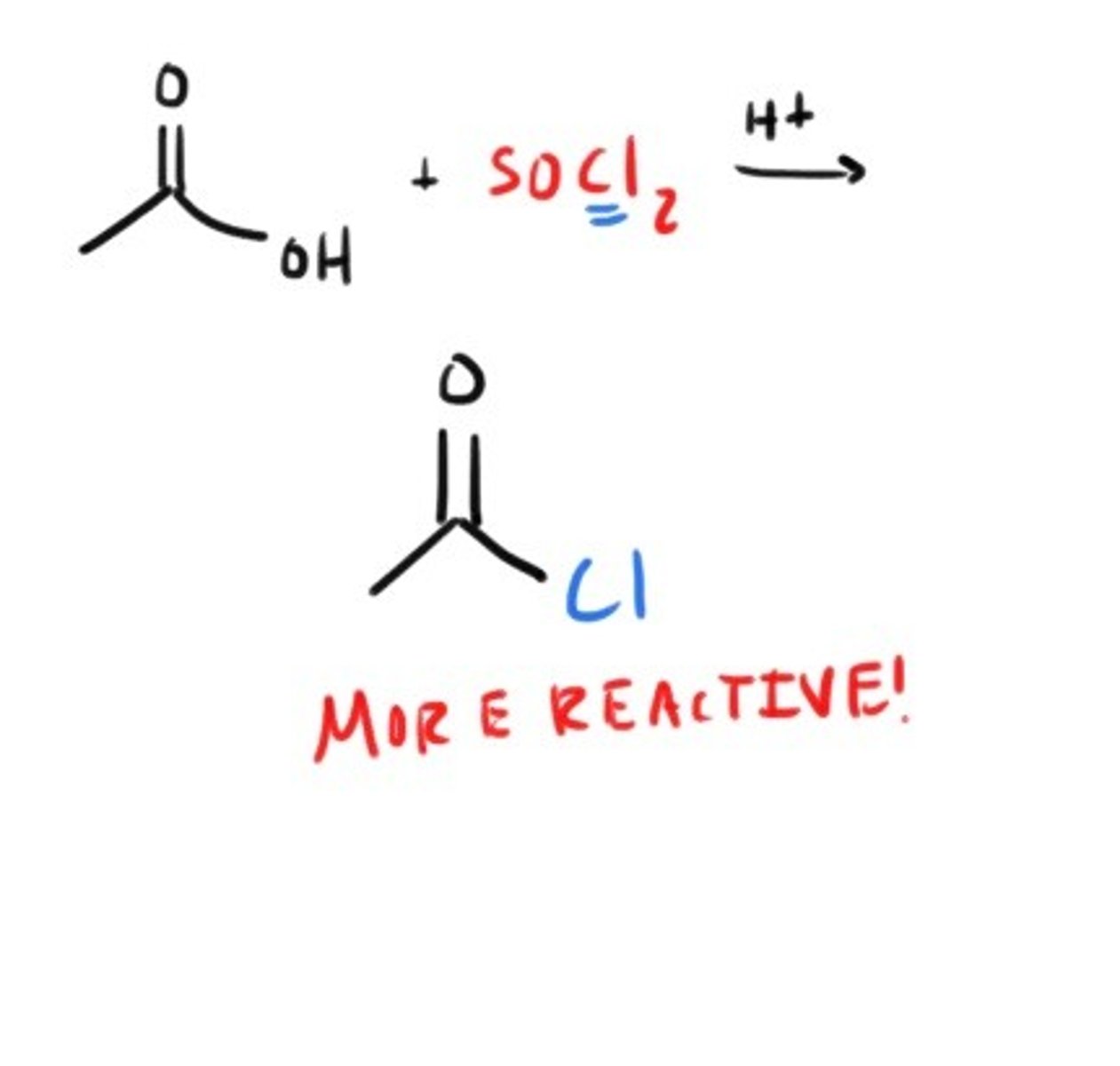
Acyl Halide from CarbAcid
When might decarboxylation occur?
When carboxylic acids are heated, they may spontaneously loose a CO₂
What is the order of reactivity for carboxylic acid derivatives?
Acyl halide > anhydrides > esters > amides
How do you get an acyl halide from a carboxylic acid?
SOCl₂
Friedel Crafts Acylation
Aromatic ring acylated through electrophillic aromatic substitution.
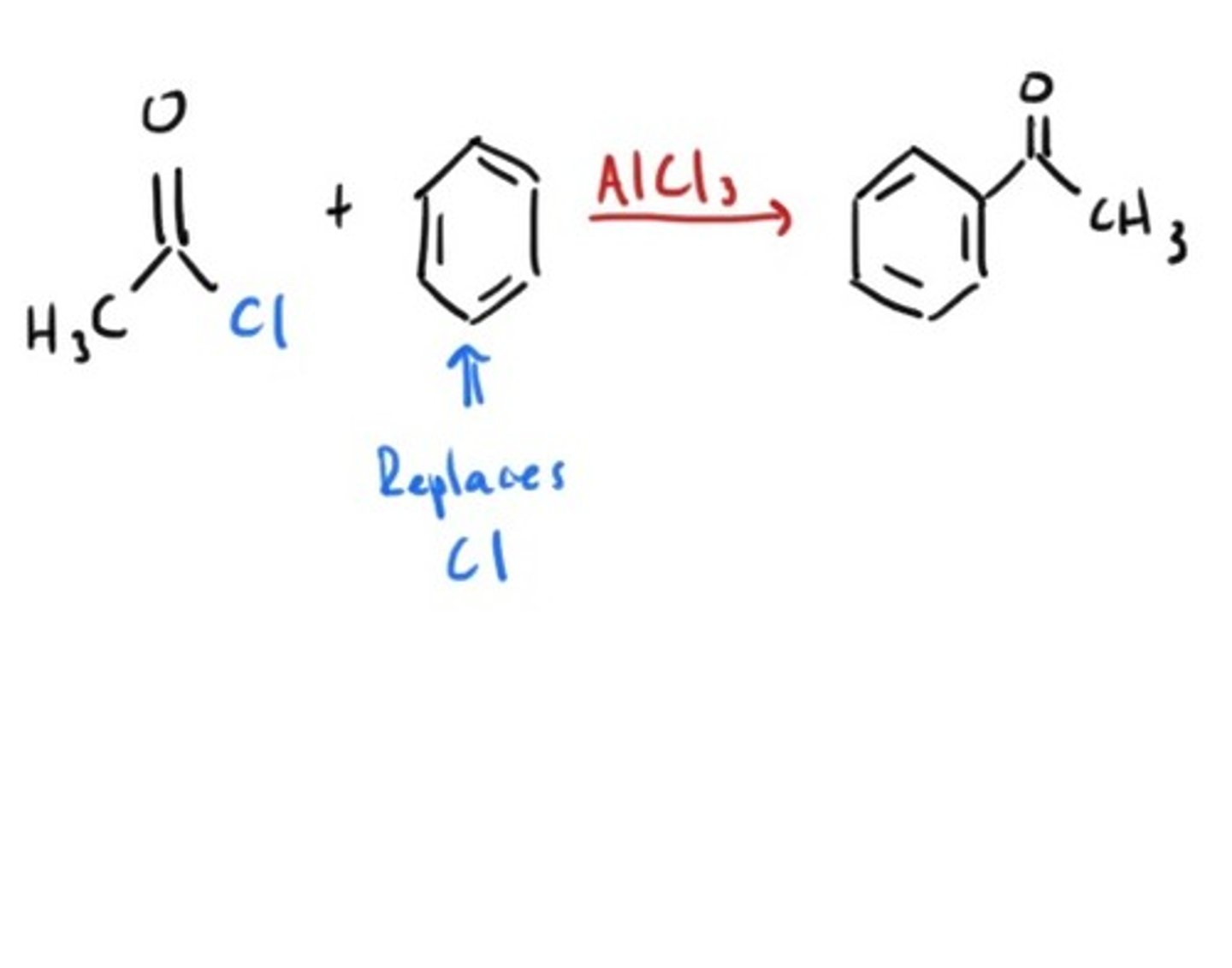
Reduction of acyl halide?
H₂ Pd/BaSO₄ Quinoline
What does acetic anydride look like?
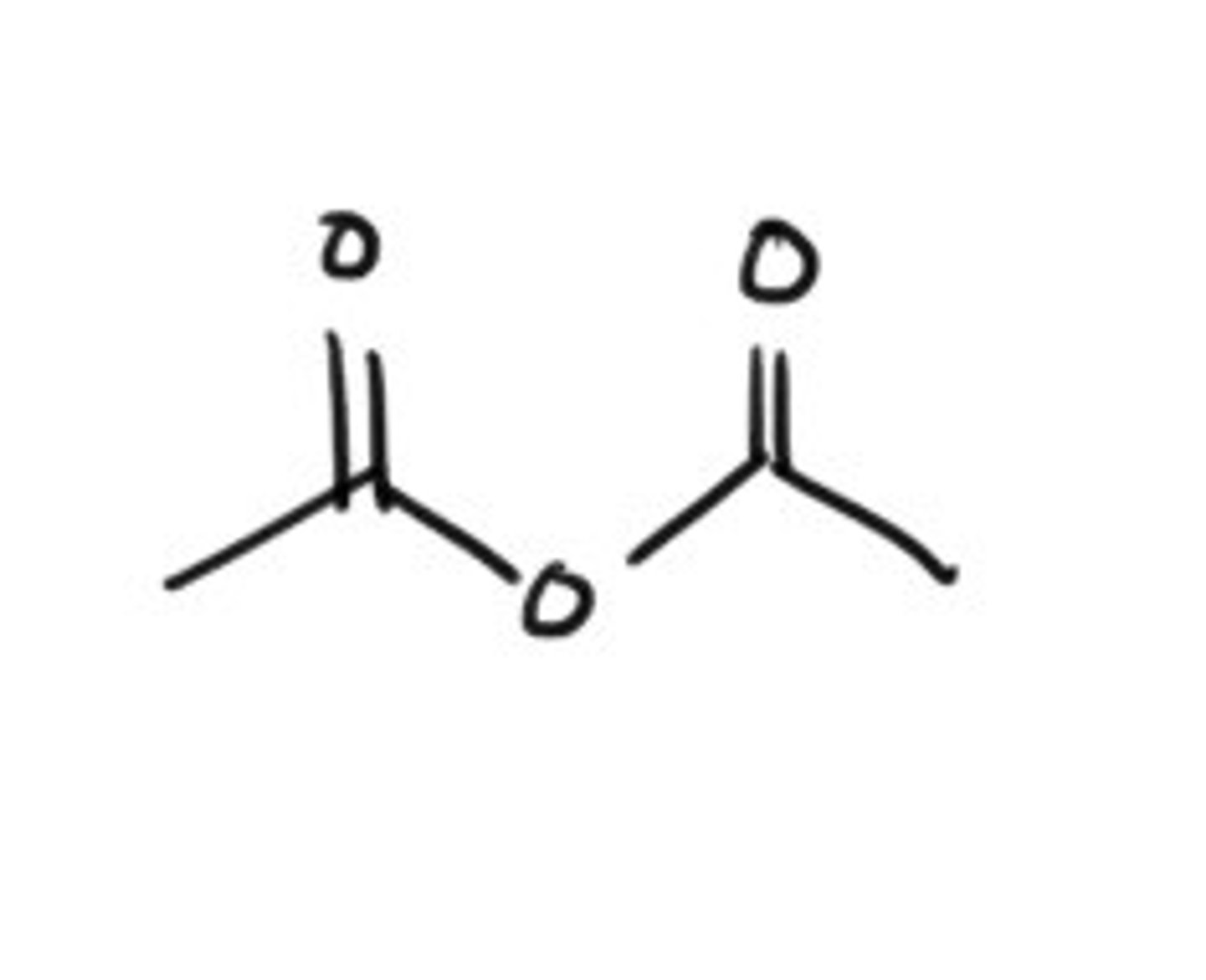
How can one make an anhydride?
React a carboxylic acid with a carboxylate salt or heat! to stabilize the carboxylic acid.
The conversion of a carboxylic acid into ANYTHING depends on what?
The nucleophile!!
When carbonyl's are attacked by hydrolysis, what does the nucleophile look like in acidic vs basic conditions?
Acidic: H₂O is attacking
Basic: OH- is attacking
In acidic conditions the carbonyl oxygen is protonated.
How can you think of Lithium Aluminum Hydride?
It is like an H- nucleophile.
Can nitrogen containing compounds form hydrogen bonds?
Yes, but the are not as strong as the hydrogen bond between H and oxygen! (Therefore lower boiling point).
Can nitrogen-containing compounds be optically active?
Usually not because of nitrogen inversion but sometimes they can be isolated if the structure hinders inversion.
How what is Gabriel Synthesis for?
Gabriel drank ammonia! OH NO!
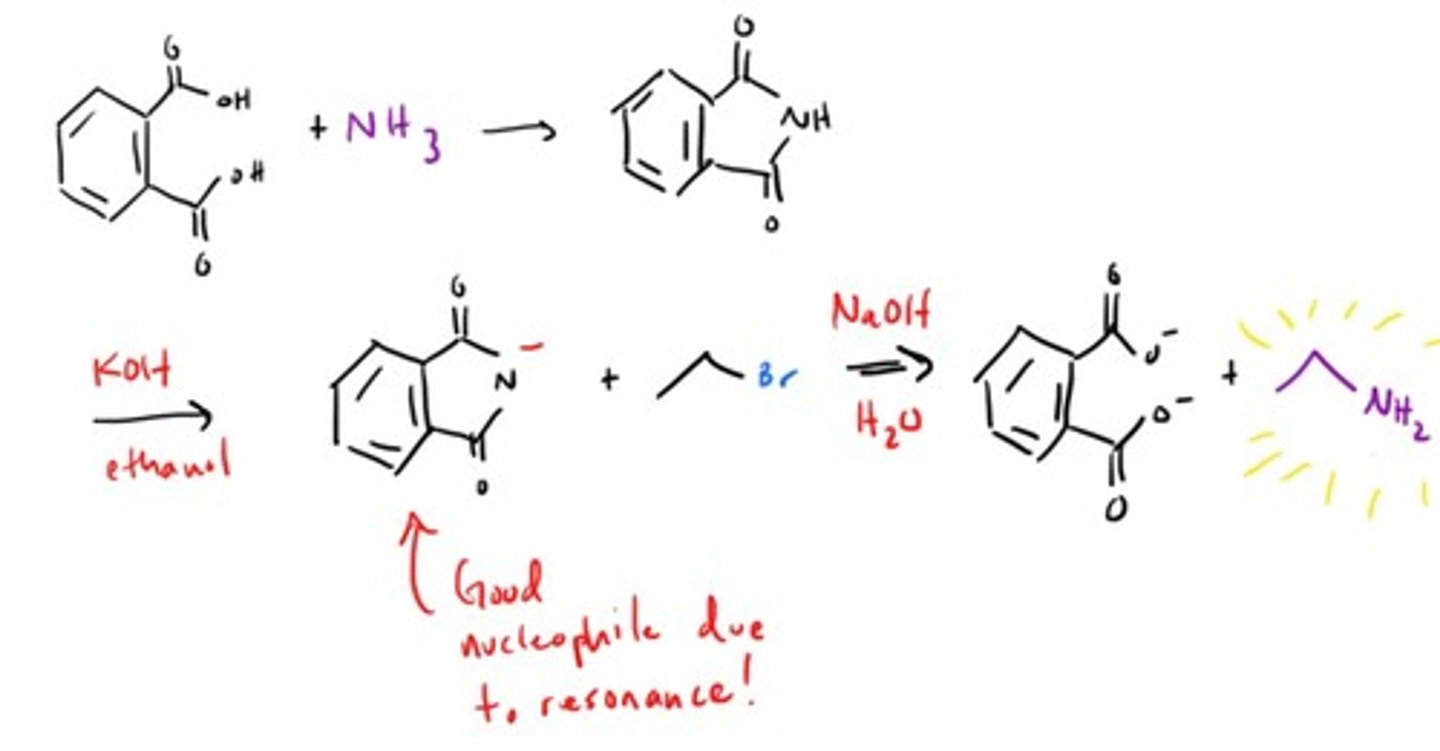
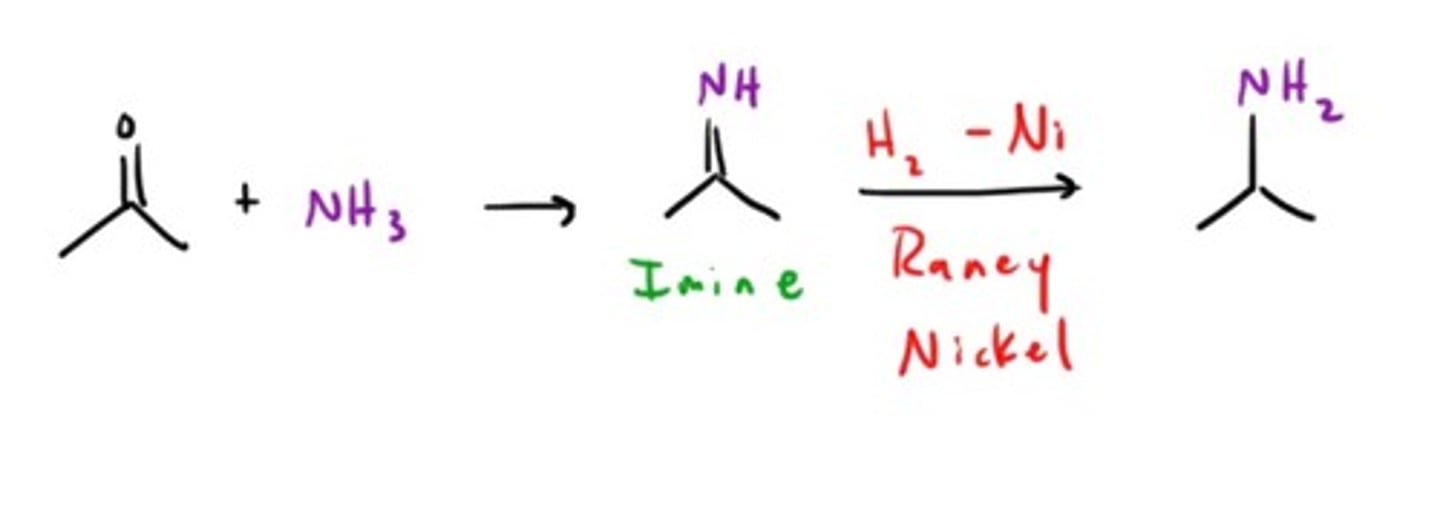
Amine from Nitro compound
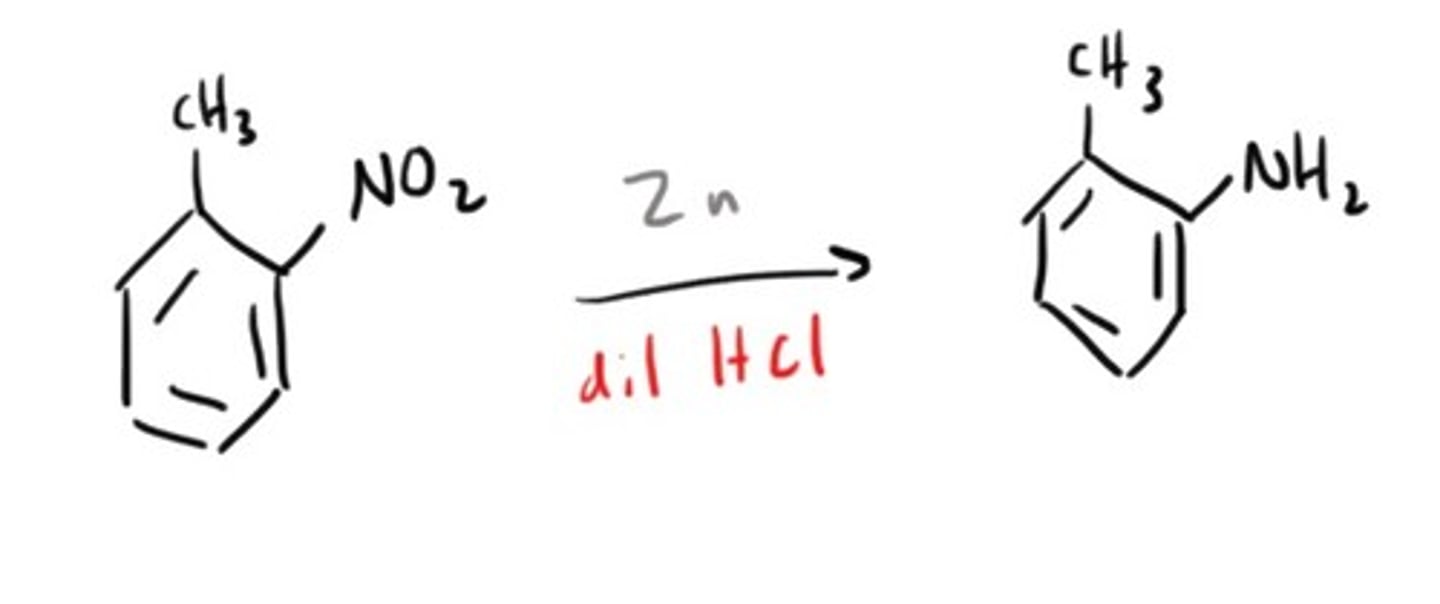
Amine from a Nitrile/Cyanide
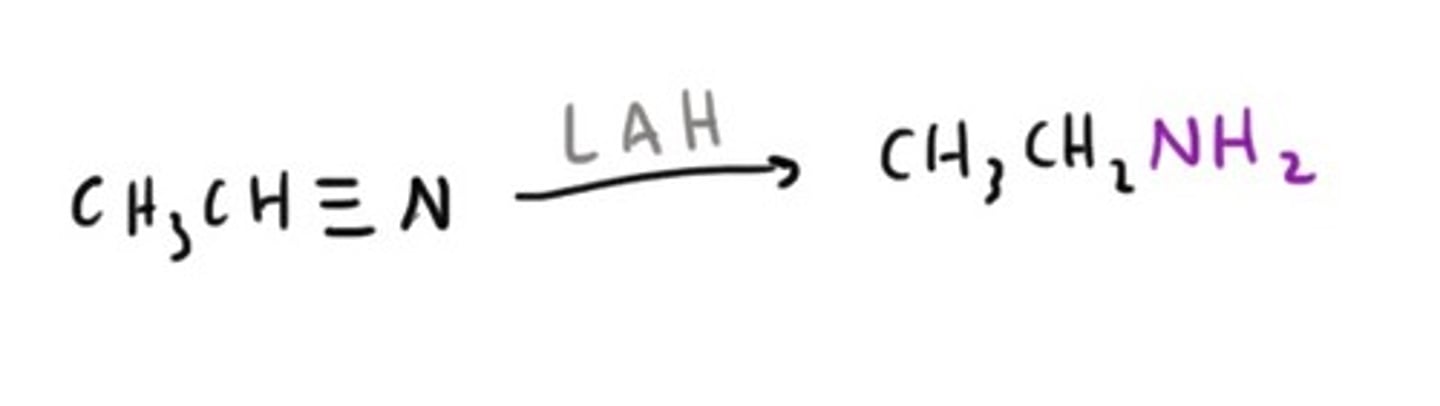
Amine from an Amide
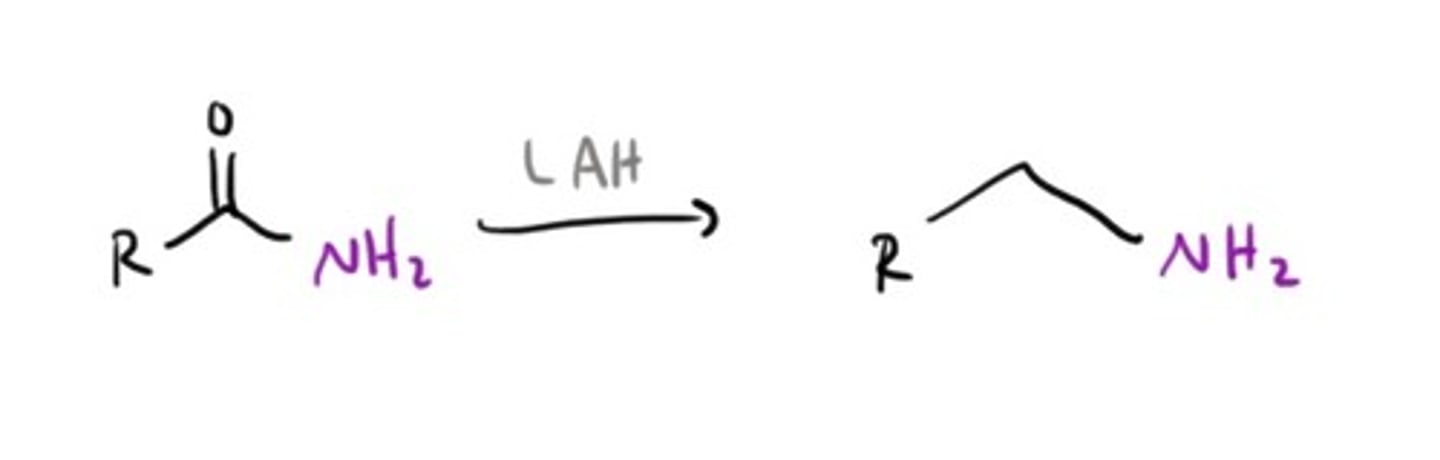
Amine from an Imine
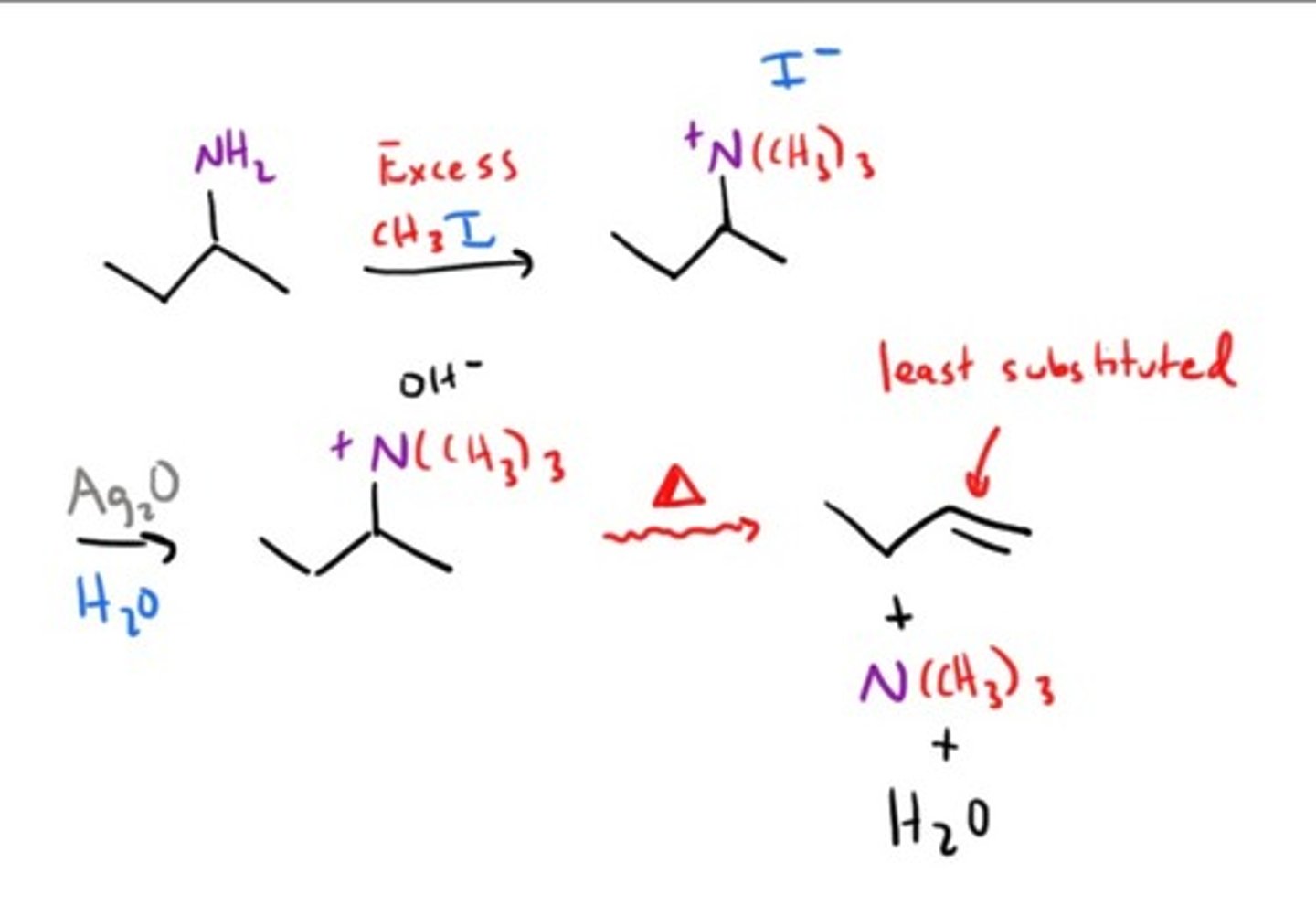
Hofmann elimination (not to be confused with hofmann rearrangement)/Exhaustive Methylation
What is hofmann rearrangement?
An amide to a primary amine (Pg 389)
What is a wash?
When you are using the extraction to remove impurities rather than separate out the desired product.
Gravity filtration vs vacuum filtration
Gravity filtration is used for when the desired product is in solution (usually hot) where as vacuum filtration has the desired product being a solid.
Why is temperature important in recrystallization?
The product needs to be soluble in high temps and insoluble in lower temperatures. IMPURITIES need to be soluble at all temperatures so that they stay in solution.
When would distillation be favored over a separatory funnel?
When two liquids are miscible, the separatory funnel cannot be used therefore distillation is more ideal.
When is vacuum filtration used?
ABOVE 150 C BOILING POINTS!
Vacuum will lower the pressure of the apparatus to ensure that the liquids do not decompensate under the extreme temperatures.
Fractional distillation
Repeated distillations on inert objects like glass beads that help separate two liquids with boiling points less than 25 C apart.
Thin-Layer Chromatography
Silica gel is polar!!
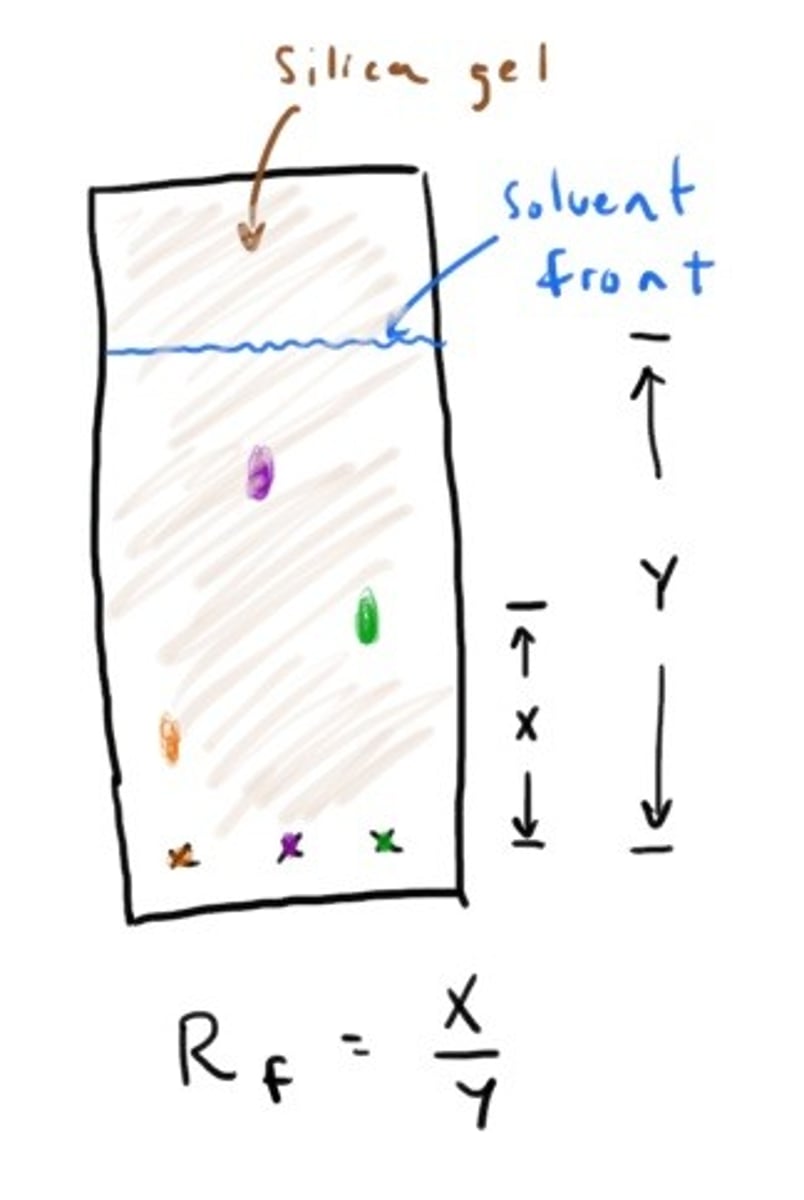
Column Chromatography
Ion Exchange Chromatography
Size-Exclusion Chromatography
Affinity Chromatography
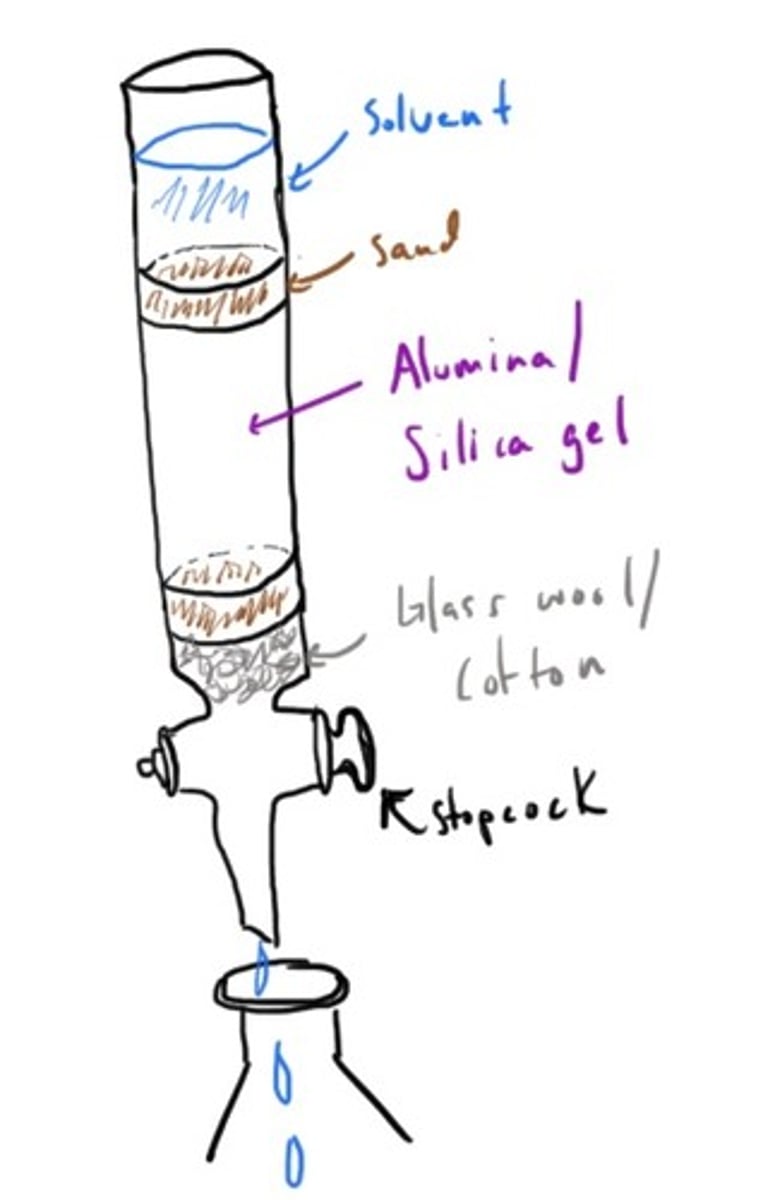
In gas chromatography what is the eluant and what is the stationary phase?
The eluant or mobile phase is a gas (He or N) and the stationary phase is the temperature-regulated column.
Retention time: How long it took for each compound to travel through the column.
How does isoelectric focusing work?
All proteins have an isoelectric point (no charge). When the molecule reaches the field that is equal to its isoelectric point, it will stop moving!
How do Agrose gel and SDS-PAGE electrophoresis separate molecules?
Based on SIZE
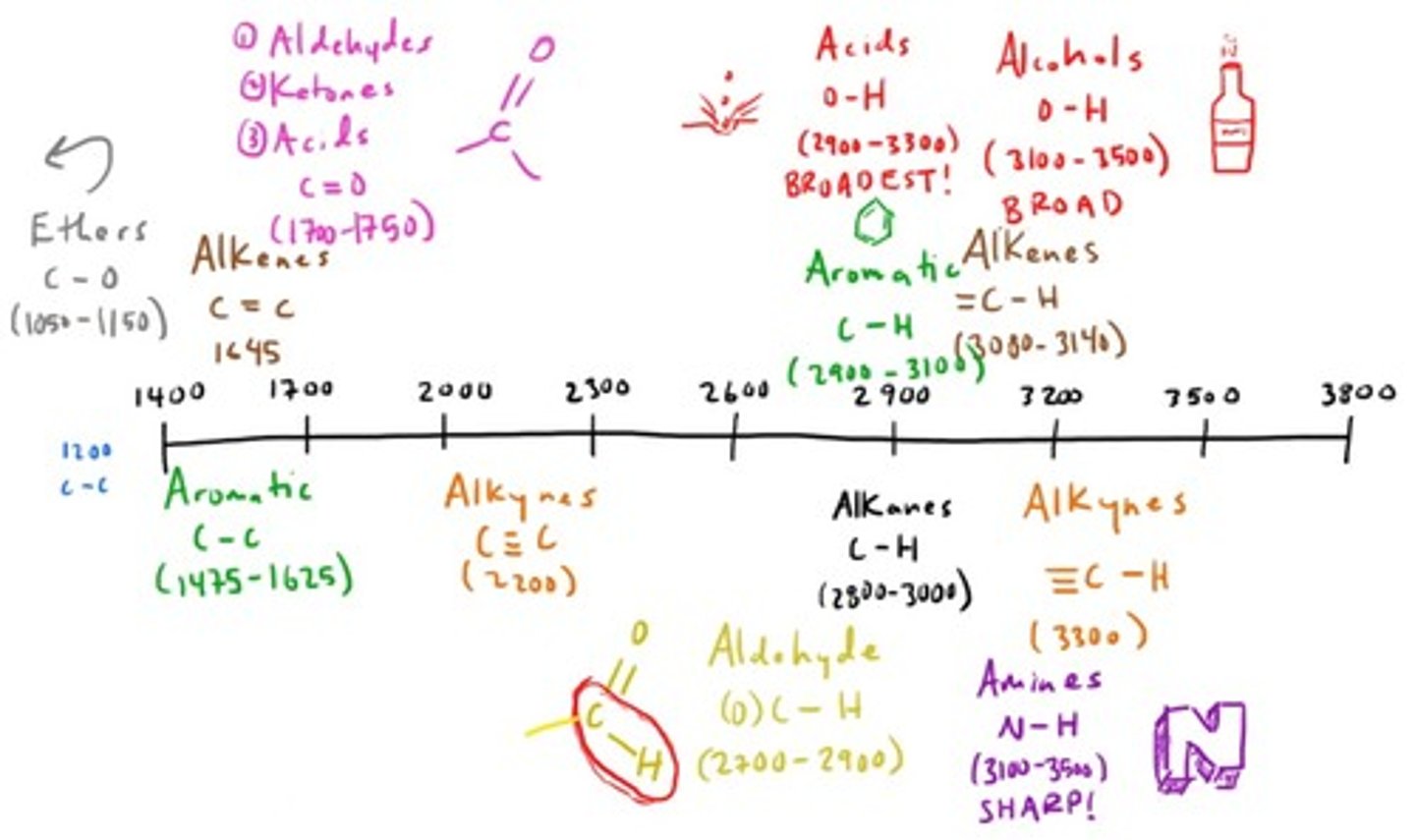
What is the purpose of Infrared (IR) spectroscopy?
Certain bonds absorb infrared light at different frequencies and this can then be measured through what is absorbed vs what is transmitted.
Used for FUNCTIONAL GROUP identification.
IR Absorption Peaks
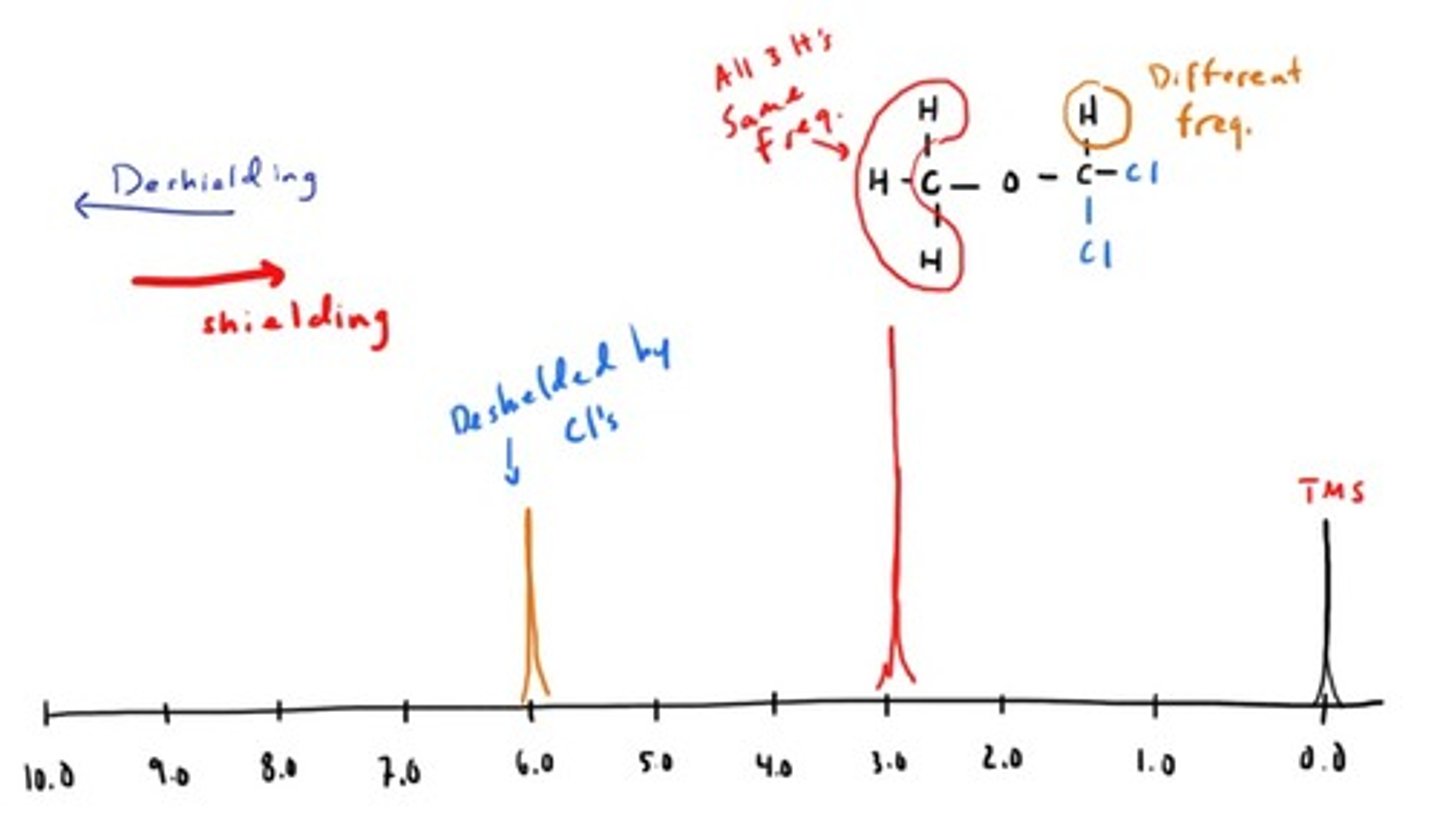
NMR