Freshwater Ecology: Midterm Study Guide
1/121
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
122 Terms
Freshwater Ecology
Study of fresh waters contained within terrestrial or continental boundaries.
Freshwater vs. Saline
Freshwater has lower salt content compared to marine or saline environments.
Groundwater vs. Surface Water
Groundwater is found underground, while surface water is on the earth's surface (e.g., lakes, rivers).
Lentic (Standing Water) vs. Lotic (Flowing Water)
Lentic refers to still waters (e.g., lakes), and lotic refers to moving waters (e.g., rivers).
List the four Aquatic Ecosystem Services
Supporting: (Processes that support basic life) Nutrient cycling and primary production.
Provisioning:(Can be extracted from nature) Resources like water, fish, and timber.
Regulating: (moderate natural phenomena) Control of climate, water purification, etc.
Cultural: (development and cultural advancement)Aesthetic, recreational, and religious values.
Hydrologic Stressors
Increased stormwater runoff
Reduced summer low flows
Altered flooding regim
Physical Stressors
Sedimentation
Unstable Stream Banks
Suspended Sediments
Migration Barriers
Stream and Wetland Fill
Thermal Pollution
Chemical Stressors
Nutrient Loading
Acid Precipitation
Acid Mine Drainage
Alkaline Mine Drainage
Toxins
Endocrine Disruptors
Biological Stressors
Exotic Species
Altered Trophic Structure
Bio-toxins (e.g., golden algae)
Reduced Energy Base
ecosystem
A functioning natural unit with interacting biotic and
abiotic components in a system whose boundaries
are determined by cycles and fluxes of energy,
materials, and organisms (e.g., Great Lakes
Ecosystem, Okeefenokee Swamp Ecosystem,
Monongahela River / Tributary Complex)
Ecosystem Structures
Biological chemical, and
physical elements in a system
Ecosystem Processes
How biological, chemical, and
physical ecosystem structures interact
Ecosystem Services
The conditions and processes through which natural
ecosystems, and the species that make them up, sustain and
fulfill human life
Why study freshwater ecology?
Human societies depend on aquatic ecosystems, and these systems are deteriorating rapidly.
Restoring ecosystems is costly; prevention is essential for sustainability.
Describe polarity between water molecules
Water molecules have a positive and negative side, which allows them to form hydrogen bonds.
How does water have a high heat capacity?
Water absorbs and retains heat, stabilizing environments.
When is water densest?
Water is densest at 4°C, impacting how it circulates in lakes.
Viscosity
the resistance to change in form
Inertia
the resistance of a body to a change in
its state of motion (objects with greater mass have greater inertia)
Reynold’s number
Measures the forces of water moving around objects (important for aquatic life).
Why is photosynthesis important for aquatic organisms?
Sunlight supports aquatic life through energy capture.
Thermal Stratification
the division of water layers based on temperature (epilimnion, metalimnion, hypolimnion).
Eplimnion
Warm, upper layer
Metalimnion (Thermocline)
Middle layer with rapid temperature change.
Hypolimnion
Cold, deep layer
Attenuation
Light diminishes as it passes through water, affecting the depth at which organisms can photosynthesize.
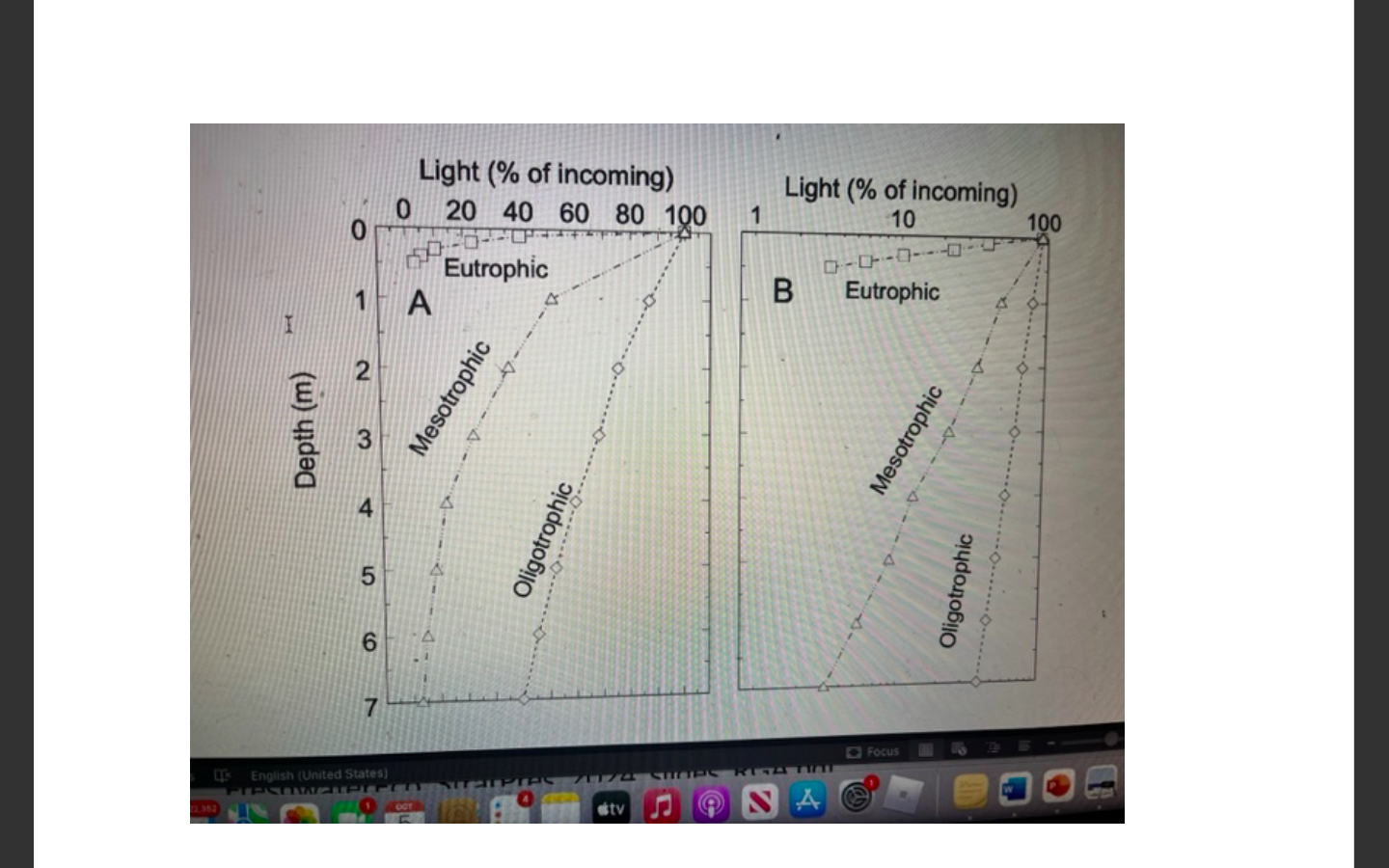
Explain what is happening in this graph
As you move from eutrophic to oligotrophic waters, the depth to which light can penetrate increases.
Eutrophic waters: Light diminishes quickly because the high levels of organic material (like algae) block sunlight.
Oligotrophic waters: Light penetrates much deeper because these waters are clearer, with less organic material.
This graph illustrates how the clarity of water affects the depth to which sunlight can penetrate, impacting how far photosynthesis can occur.
Compensation Depth
the depth in a water body (like a lake) where the amount of light available is just enough for photosynthesis to produce oxygen at a rate that equals the consumption of oxygen by respiration. In other words, it's the depth where photosynthesis = respiration.
residence time
Within the hydrologic cycle, the term "residence time"means the amount of time a material spends in an ecosystem compartment. This matters to people becaus eresidence time can determine how quickly biota can be washed out of water.
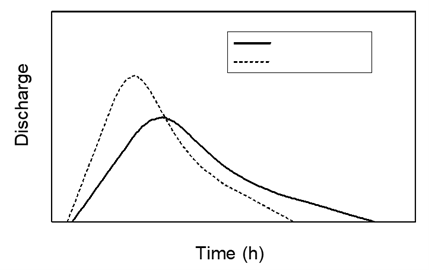
Looking at the hydrograph below, which of the two lines (either solid or dotted line) would represent a more urbanized stream or river area? Explain why briefly. (3 points)
This is because the graph shows a faster rise and fall in discharge over time, meaning the water flows into and through the stream or river very quickly, likely because of fast runoff through urban areas with lots of pavement, parking lots, roads, etc.
Hydrologic Cycle
continuous movement of water through evaporation, precipitation, and runoff.
Aquifer
A body of permeable rock that can store and transmit groundwater.
Recharge
The process where water is added to an aquifer.
Freshwater Stress
Occurs when demand for freshwater exceeds supply.
Runoff Processes
Hortonian Overland Flow: Water flowing over land when rain exceeds infiltration.
Baseflow vs. Stormflow:
Baseflow: Groundwater flow sustaining rivers during dry periods.
Stormflow: Rapid flow from rain or snowmelt.
Types of Streams
Ephemeral: Flows only after precipitation.
Intermittent: Flows during certain times of the year.
Perennial: Flows year-round.
Stream Discharge
volume of water flowing past a fixed location over some period of time (usually quantified as cubic feet per second or cubic meters per second)
Fluvial Geomorphology
Study of landform structure and changes over time resulting from fluvial processes (water flow and sediment transport).
Watershed
The land area that channels rainfall to rivers and lakes.
Channel Morphology
Refers to the shape and structure of a river or stream channel, influenced by sediment supply, water flow, and vegetation.
Habitat
A place where an organism or a community of organisms lives, including all living and non-living factors or conditions of the surrounding environment
Why do lakes tend to be aggregated (grouped together)?
This clustering happens because most lakes are formed by catastrophic events like:
Tectonic activity: Movement of the Earth's plates.
Volcanic eruptions.
Glacial activity: Movement of glaciers carving out basins that become lakes.
Tectonic Basins
Graben Lakes: Form in areas where the Earth's crust stretches and creates a depression (e.g., Lake Baikal).
Horst Lakes: Formed by blocks of land being uplifted (e.g., some African Rift Valley lakes).
Climatic causes for lake origins
Sea Level Rise: Rising sea levels can flood areas, forming lake
Earthslide
Large landslides can block rivers or create depressions, forming lakes.
Volcanic Activity
Crater Lakes: Form in the crater of an extinct or dormant volcano.
Maar Lakes: Created by explosive volcanic activity.
Calderas: Formed when a volcano collapses after an eruption, leaving a large crater.
Glacial Activity
Ice Scour Lakes: Carved out by glaciers.
Cirque Lakes: Form in bowl-shaped depressions created by glaciers at the head of valleys.
Piedmont Lakes: Form where glaciers flow out onto flat plains.
Lakes associated with glacial moraines
Morainal Damming: Lakes formed when glacial debris (moraines) block rivers.
Kettle Lakes: Created by blocks of ice left behind by retreating glaciers that melt and form depressions.
River Activity
Oxbow Lakes: Formed when a meandering river cuts off a loop, leaving a U-shaped lake.
Lakes formed from dissolved limestone
Dissolution Lakes: Form when limestone dissolves, creating sinkholes that fill with water.
Biogenic Lakes
Lakes created by living organisms:
Beaver Dams: Beavers build dams that create lakes.
Human Activity: Lakes formed by human construction, like reservoirs.
Lake Morphology
the physical characteristics of lakes, including their shape and size.
Bathymetric Maps
These maps show the underwater contours (depths) of a lake, similar to a topographic map.
Morphometric parameters
These are measurements that describe the size and shape of a lake
Max length (l)
The longest distance across the lake
Max width (b)
The widest distance across the lake
Volume (v)
The total amount of water in a lake
Area (A)
The surface area of the lake.
Max depth (zm)
Deepest point in a lake
Mean Depth (zm)
The average depth of a lake
Shoreline Development (DL)
A measure of how "irregular" the lake's shoreline is. High DL means a more irregular, complex shoreline.
What are lake habitats defined by light and compensation point?
Littoral Zone: The shallow area near the shore where light penetrates to the bottom, allowing plants to grow.
Pelagic Zone: The open water area where light still penetrates but does not reach the bottom.
Profundal Zone: The deep area of the lake where light cannot reach and no photosynthesis occurs.
What are lake habitats defined by temperature and density?
Epilimnion: The warm, upper layer of water in a stratified lake.
Metalimnion (Thermocline): The middle layer where temperature changes rapidly with depth.
Hypolimnion: The cold, deep layer of water where the temperature is uniform and low.
Reservoir
artificial lakes, and they are divided into zones based on flow and temperature patterns
flux
transfer of molecules from one specific reservoir to another over a period of time
Riverine zone
The area near the river’s inflow, where water is fast-moving and narrow.
Transition Zone
The middle area where water flow slows down and sediment begins to settle.
Lacustrine Zone
The area near the dam that behaves more like a natural lake, with slow-moving water.
Tailwater Zone
the section of a river immediately downstream of a dam or reservoir. It is influenced by the release of water from the dam, which can affect both the flow and temperature of the water in this area.
Tailwater Zone characteristics
Flow Regimes: Patterns of water movement, affected by the release from dams.
Thermal Regimes: Temperature changes influenced by water released from the reservoir.
Basic elements of water
Hydrogen (H2) and Oxygen (O2):
Suspended Inorganic Particulate Matter (SIPM)
non-living particles such as slit or clay
Suspended Organic Particulate Matter (POM)
Organic particles, often derived from decomposed plants or organisms.
Dissolved Major Ions
Cations (Positive Ions): Calcium (Ca²⁺), Magnesium (Mg²⁺), Sodium (Na⁺), Potassium (K⁺).
Anions (Negative Ions): Bicarbonate (HCO₃⁻), Carbonate (CO₃²⁻), Sulfate (SO₄²⁻), Chloride (Cl⁻).
Dissolved nutrients
Nitrate (NO₃⁻): Essential for plant growth but can cause eutrophication in excess.
Phosphate (PO₄³⁻): Critical for photosynthesis but can also contribute to algal blooms if overabundant.
Dissolved Organic Matter
Organic molecules in solution, often originating from plant material and decomposed organisms.
Dissolved gases
Oxygen (O₂): Necessary for respiration in aquatic organisms.
Nitrogen (N₂): Inert gas, can become biologically available through nitrogen fixation.
Carbon Dioxide (CO₂): Used in photosynthesis, influences water pH.
Dissolved Metals
Metals in trace amounts that vary with pH, often naturally occurring but can be toxic at higher concentrations. Examples:
Iron (Fe), Aluminum (Al), Manganese (Mn), Zinc (Zi), Nickel (Ni), Cadmium (Cd), Chromium (Cr), Barium (Ba), Copper (Cu), Selenium (Se).
Surface water chemistry
Determined by interactions between dissolved substances (gases, ions, nutrients) and surrounding geology, biology, and human activities. Weathering of rocks and atmospheric inputs (e.g., rainfall) significantly affect water chemistry.
Dissolved Oxygen (DO)
Importance: Oxygen is crucial for fish and other aquatic organisms to survive.
Low DO Levels: Can cause "fish kills" and stress aquatic life, particularly during warm conditions when oxygen dissolves poorly in water.
Effects on Fishes: Insufficient oxygen can lead to impaired respiration, decreased metabolic rates, and death in extreme cases.
How do aquatic organism breathe?
Aquatic organisms, such as fish, extract oxygen from water using gills. Gills allow oxygen (O₂) to diffuse from the water into the blood, just as lungs do in terrestrial organisms.
Oxygen Saturation vs. Temperature and Pressure
Oxygen Saturation: The maximum amount of oxygen that water can hold at a given temperature and pressure.
Temperature: Cold water holds more dissolved oxygen than warm water. As water warms, its capacity to hold oxygen decreases.
Pressure: Increased pressure (e.g., deeper water) allows water to hold more dissolved oxygen.
Photosynthesis and Respiration
Photosynthesis: Aquatic plants and algae use sunlight to convert carbon dioxide (CO₂) and water into oxygen and glucose.
Respiration: Both plants and animals use oxygen to break down food (glucose), releasing carbon dioxide and energy.
Photosynthesis vs. Light (P-I) Relationships
P-I Curve: Shows how the rate of photosynthesis (P) increases with light intensity (I). At low light levels, photosynthesis is limited. It increases with light until it reaches a saturation point where further light does not increase the rate.
Photosynthesis vs. temperature
Photosynthesis is temperature-dependent. Too low or too high temperatures can reduce the rate of photosynthesis, as enzymes responsible for the process are sensitive to temperature changes.
Temporal Variation in oxygen (Lake vs. stream)
Lakes: Oxygen levels in lakes vary more dramatically over time due to temperature stratification and slower water movement.
Streams: Oxygen levels in streams are more consistent due to continuous water flow, which increases aeration and mixing.
Variation in Oxygen with water depth
Orthograde Oxygen Curve: Occurs in deep lakes with uniform oxygen distribution, typically in cold, unproductive lakes.
Clinograde Oxygen Curve: Common in nutrient-rich lakes (eutrophic), where oxygen decreases with depth due to decomposition at the bottom.
Fish temperature preferences
Fish species have different temperature preferences (cold, cool, or warm water). As water temperature increases, their oxygen requirements also increase, but oxygen availability decreases, creating stress
Summer fish kills
Occur when hot weather reduces DO levels in shallow lakes and ponds. Warm water holds less oxygen, and respiration and decomposition rates increase, depleting available oxygen.
Winter fish kills
Occur in frozen-over lakes where sunlight cannot penetrate the ice. Photosynthesis slows or stops, while respiration continues, leading to oxygen depletion under the ice.
The temperature/ oxygen squeeze
As water temperatures rise, the combined effects of decreased dissolved oxygen availability and increased oxygen demand by aquatic organisms create challenging conditions for survival, potentially leading to shifts in species distribution and health of aquatic communities.
Concentration
Measure of components in water, applicable to both dissolved and particulate materials
Measurement: Expressed in mg/L, which equals parts per million (ppm).
Total Suspended Solids (TSS)
The concentration of all organic and inorganic particles suspended in water.
Total Dissolved Solids (TDS):
The concentration of all dissolved inorganic and organic substances in water.
Natural Sources: Primarily from the weathering of rocks.
Salinity
The concentration of dissolved inorganic substances (salts) in water.
Calculation for salinity
Salinity = TDS - dissolved organic substances.
Human Sources: Include agricultural runoff, sewage treatment, mining, and urban runoff.
Water Quality Standard: 500 mg/L is a guideline for palatability in drinking water.
Hardness
The concentration of calcium (Ca²⁺) and magnesium (Mg²⁺) salts in water.
Soft Water: < 50 mg/L; typically drains from acid igneous rock.
Hard Water: > 50 mg/L; typically drains from calcareous rock.
Conductivity
Measure of the electrical conductance of water, expressed in μS/cm.
Relation to TDS: Increases with ionic concentration and serves as an indirect measure of TDS.
pH
A measure of how acidic or basic water is, based on the concentration of free hydrogen ions (H⁺).
Amphiprotic Nature:
Water can act as both an acid (donates protons) and a base (accepts protons)