Esters and Esterification
1/33
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
34 Terms
what happens in esterification?
Is when a carboxylic acid reacts with a primary/secondary alcohol to produce an ester and water
What are the 2 conditions for esterification to happen?
Need an acid catalyst (e.g phosphoric acid)
Heat under reflux
What does heating under reflux mean?
Heating the reaction mixture at a constant temperature for a long time, and condensing any vapors being produced
Explain why phosphoric acid is used in esterification
To be a catalyst
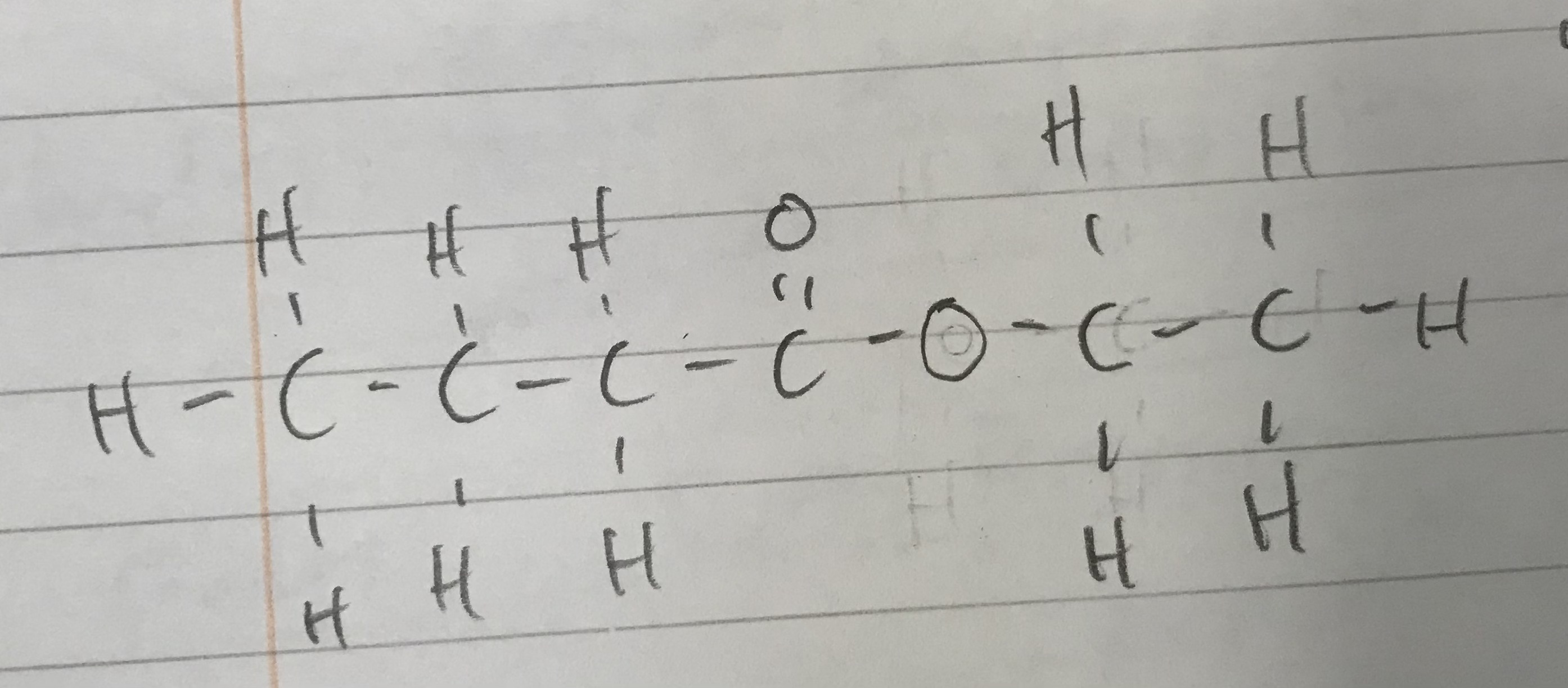
Name this ester according to IUCPAC rules
Ethylbutanoate

What are the steps to naming an ester?
Name alcohol first
Then count the carbons in the longest carbon chain
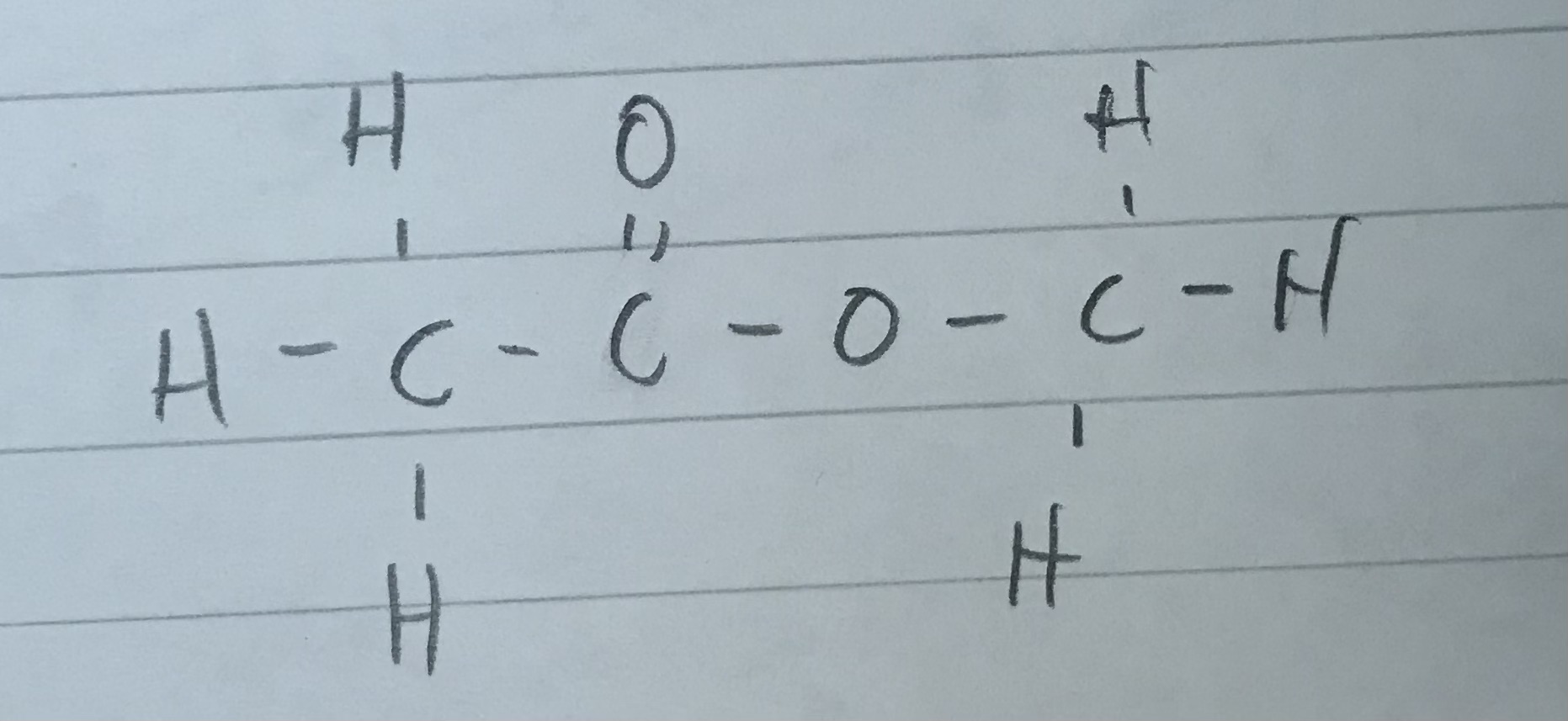
Name this ester
MethylEthanoate
What is the suffix for esters?
-oate
What happens when water is added to an ester (methylethanoate) ?
Hydrolysis occurs
And a carboxylic acid and an alcohol is produced
(Ethanoic acid and Methanol)
What happens when an alkaline (e.g SodiumHydroxide) is added to an Ester?
A salt and acid is produced
Give structural formulas for the product/s produced in the reaction between ethanol and ethanoic acid. And state the type of reaction it is

give the reagents and conditions to produce ethanoic acid from ethene
Addition- from ethene to ethanol
Steam and an acid catalyst (sufuric or phophoric acid)
Reflux- from ethanol to ethanoic acid
excess acidified potassium dichromate
What is the name of the carboxylic acid used to create Methyl ethanoate?
What is the name of the alcohol used to create it?
ethanoic acid and methanol
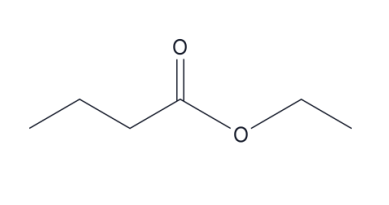
Name this compound
ethyl butanoate
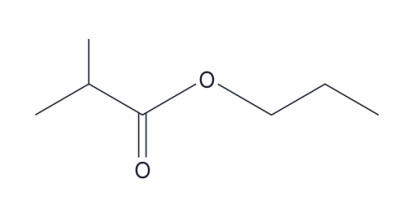
name this compound
propyl 2-methyl propanoate
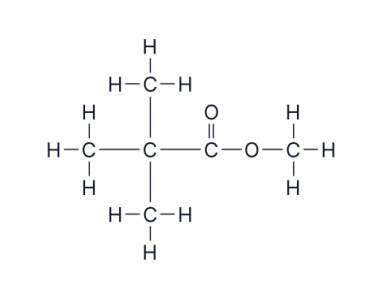
name this compound
methyl 2,2-dimethylpropanoate
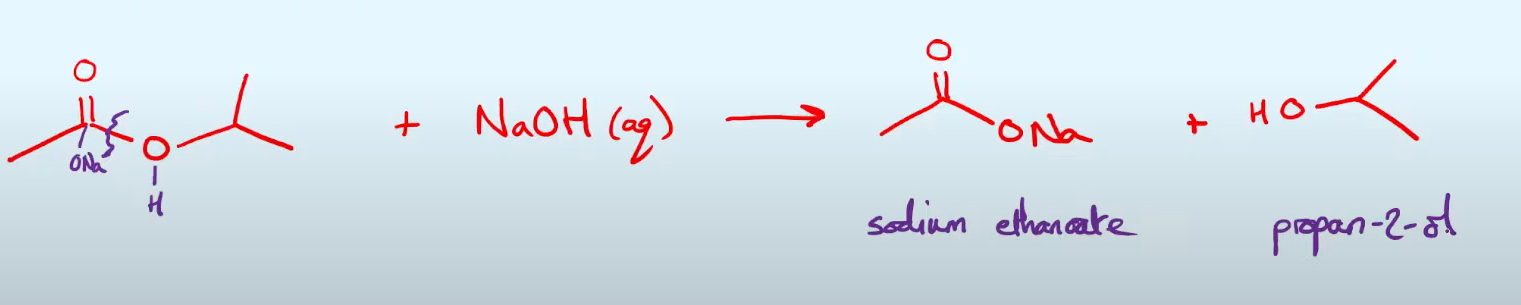
give the equation in skeletal form of the Base hydrolyisis of methyl ethyl ethanoate
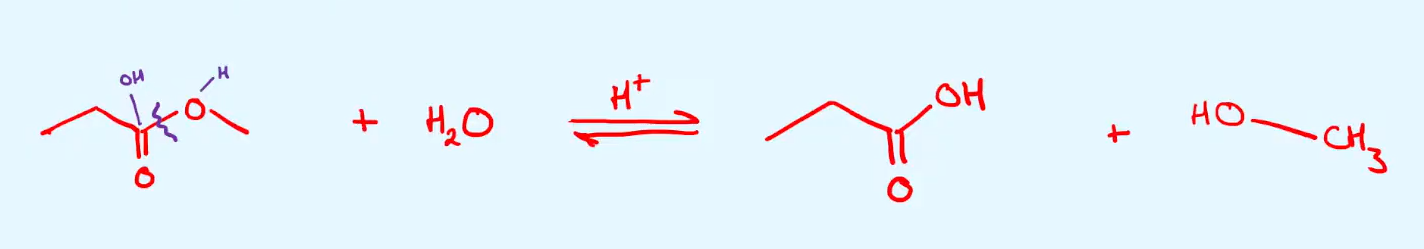
give the equation in skeletal form of the acid hydrolysis of methyl propanoate
give one similarity and 2 differences of acid base hydrolyis and base hydrolysis of esters
both produce an alcohol
acid base hydrolysis produces a carboxylic acid
base hydrolysis produces a carboxylate salt
Acid hydrolysis is a reversible reaction
Base hydrolysis is irreversible
Acid hydrolysis needs a Acid catalyst
Base hydrolysis needs base e.g NaOH
Give the reactants and products and the reagents and conditions needed in Acid hydrolysis of Esters
ester+water → carboxylic acid + alcohol
need an acid catalyst
Give the reactants and products and the reagents and conditions needed in Base hydrolysis of Esters
ester+NaOH (aq) → carboxylate salt + alcohol
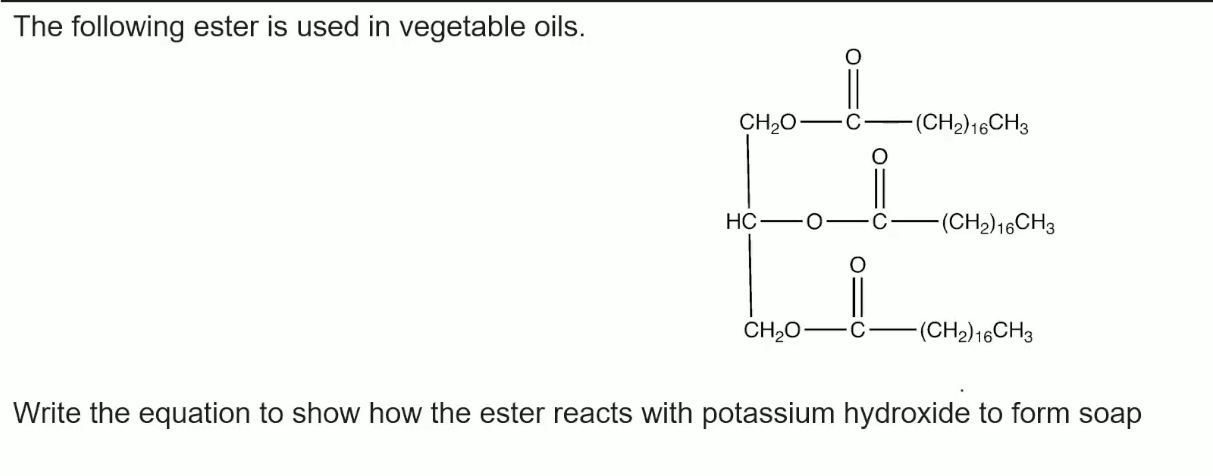
Give the displayed formula of the products in the base hydrolysis of Glycerol
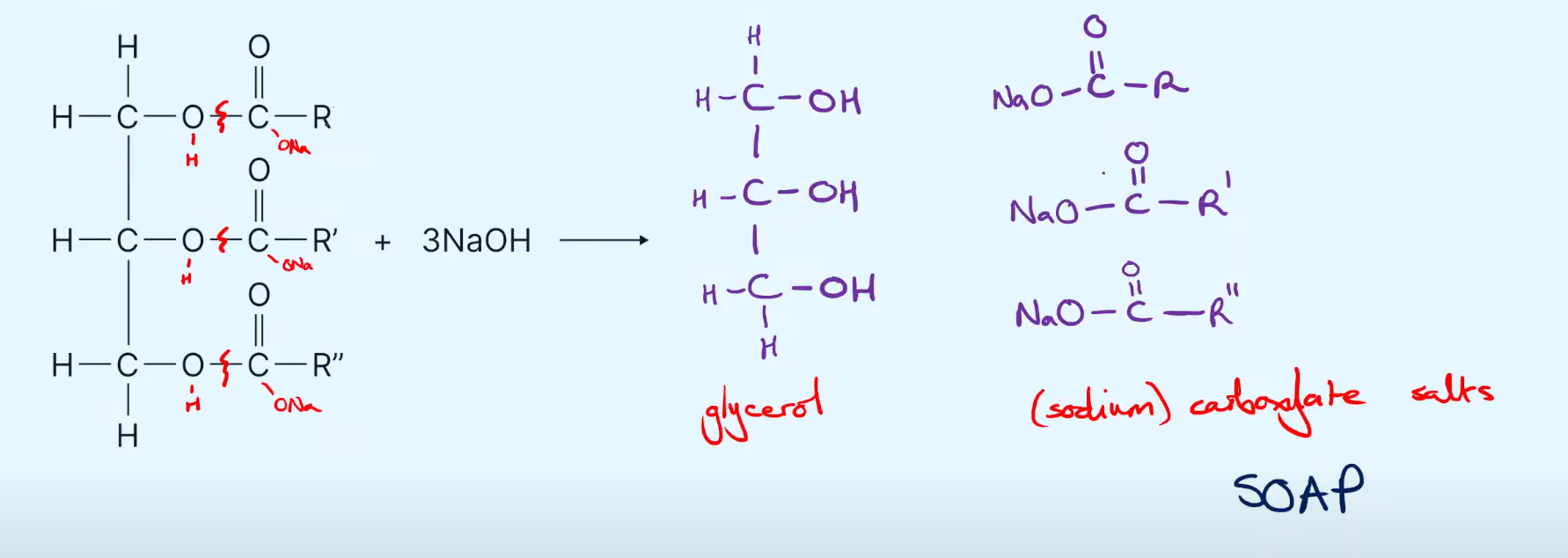
Give one use of sodium carboxylate salts
why are they used to make it
making Soap (Saponification)
because they are both hydrophilic and hydrophobic
attracted to fats and water, able to emulsify fats- Saponification
explain what type of resction Biodiesel is created and what it’s created from
transesterification using triglycerides and methanol
What are the reagents and conditions needed for transesterification of Biodiesel
Catalyst-Base catalysts-Hydroxides
vigorous mixing
tryglycerides
alcohol (excess)
Heated
Base hydrolysis is more common for the transesterfication process in industry, than acid hydrolysis. Suggest why.
It doesn't produce corrosive acidic fumes
define Biodiesel
a mixture of methyl esters
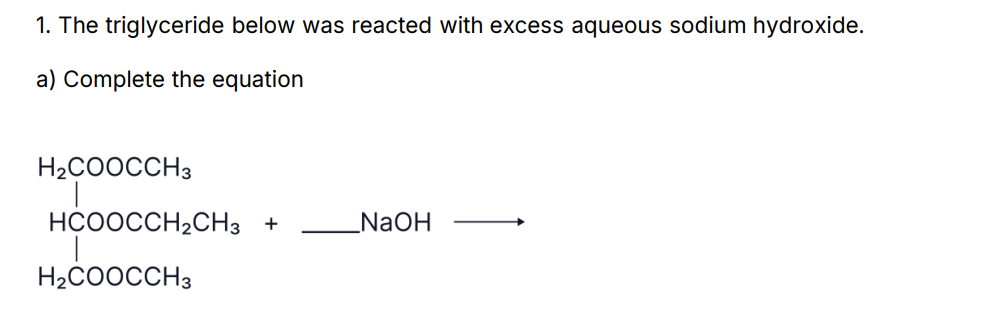
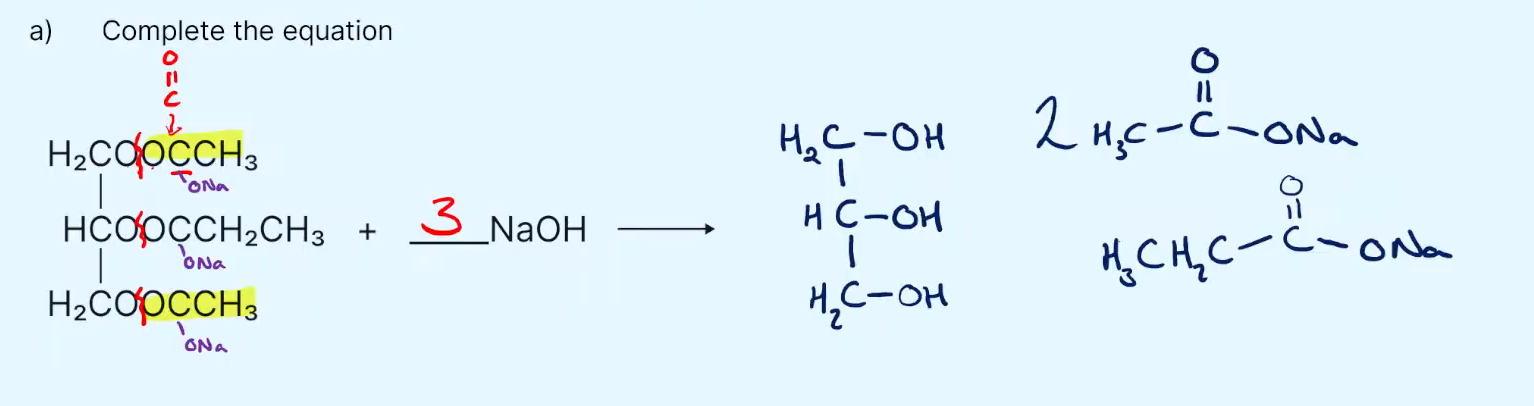
In ester hydrolysis which bond breaks?
the C-O single bond
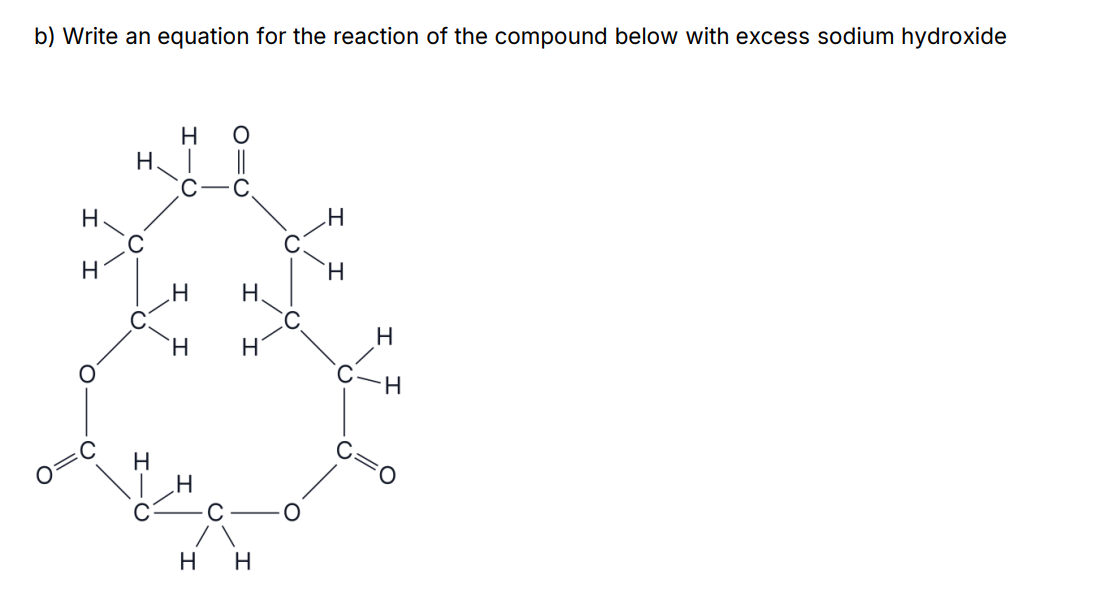
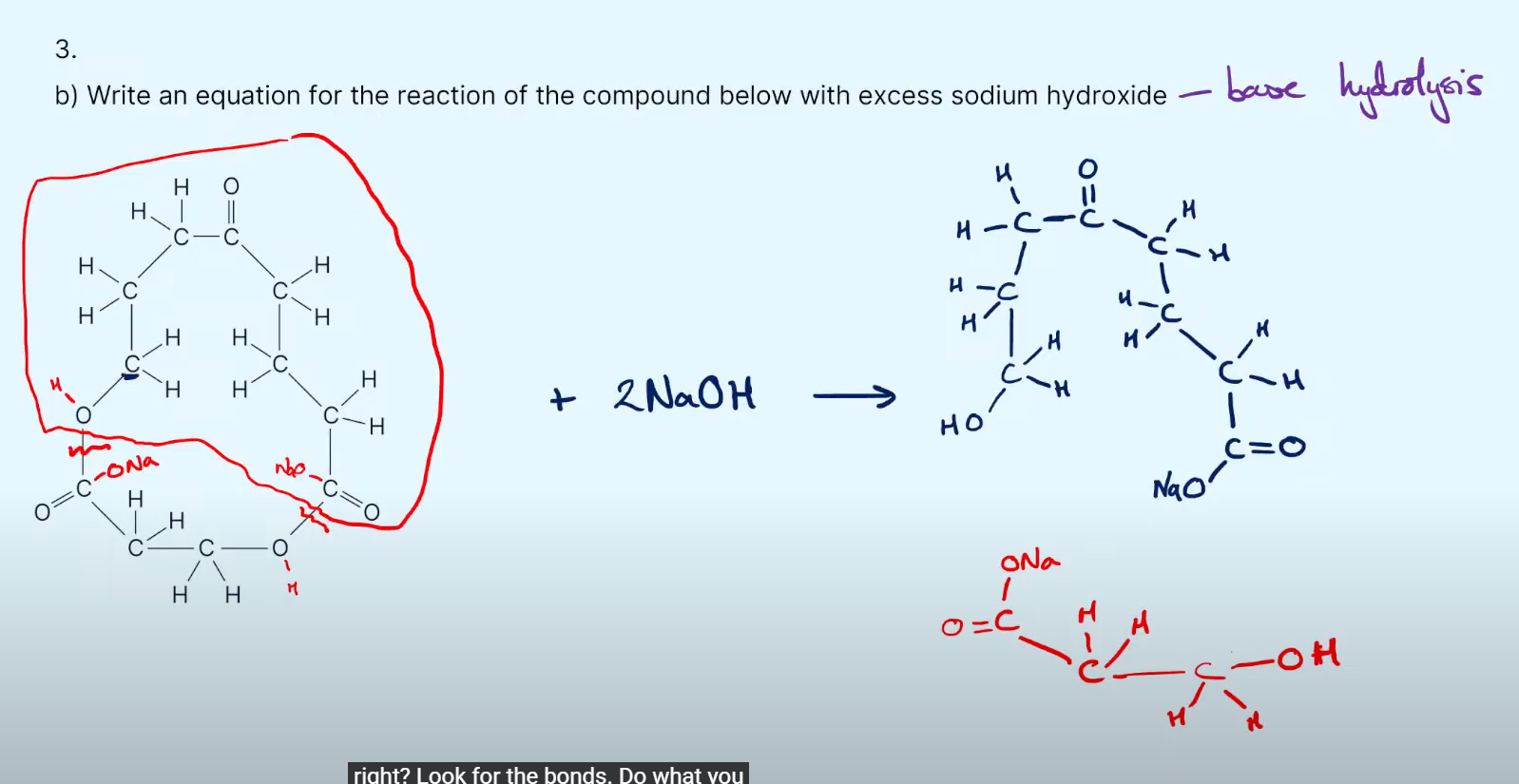

55%
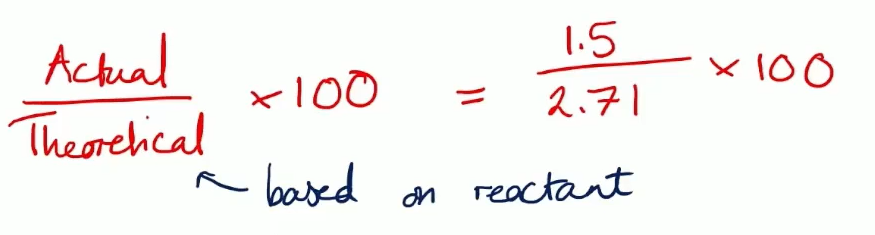
how do you name a carboxylate salt?
metal+alkan+ oate
e.g sodium methanoate
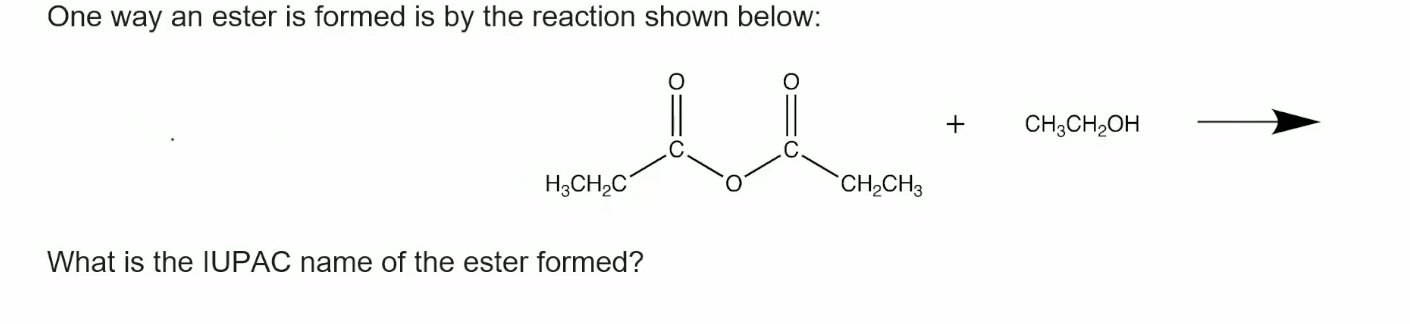
ethyl propanoate