16. Partial pressure and the diffusion of gases
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
5 Terms
is diffusion of substances really governed by concentration?
no, particles don’t really diffuse from high to low concentration. they diffuse from high to low chemical potential (a measure of energy)
in biology, we usually talk about the diffusion of dissolved solutions within a liquid solvent, and in this case, yes, areas of higher concentration always have higher chemical potential
however, this is not always true. for instance, in the respiratory system, the gasses dissolve into a liquid solvent
what is a more accurate measure of chemical potential for gases like O2 and CO2?
partial pressure (P) is the amount that a specific gas contributes to the total pressure of a compartment
gaseous solutes always diffuse from regions of high partial pressure to regions of low partial pressure
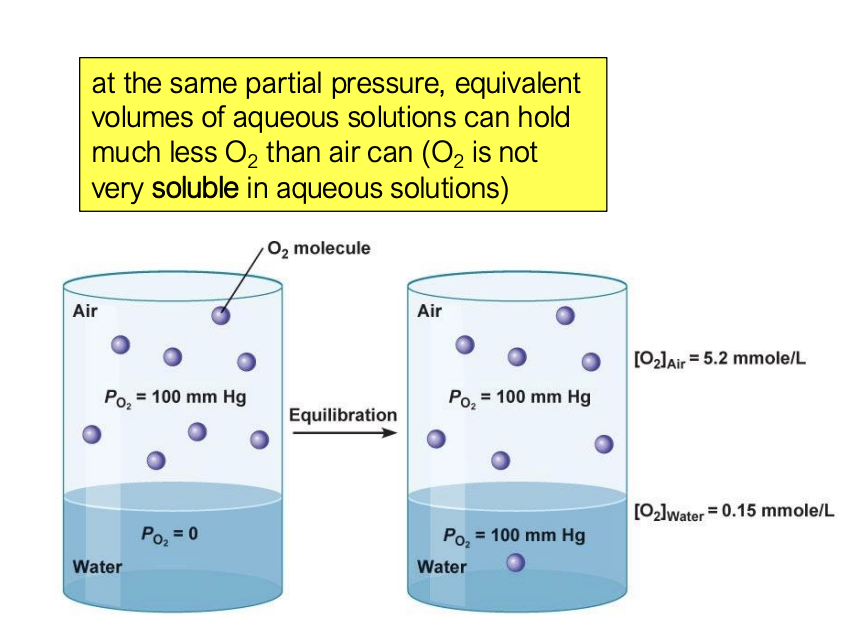
imagine you have a closed beaker with water and then you open the beaker. when will the oxygen level in the air of the beaker and the water in the beaker be equilibrium?
it will be in equilibrium when the partial pressures are the same, NOT when the concentrations of oxygen are the same
O2 is not very soluble in aqueous solutions, so water can hold much less O2 than the air can
what is the updated fick’s law of diffusion for respiratory systems?
change concentration to change of partial pressure
the greater the partial pressure difference between the air in the lungs and the blood in your pulmonary capillaries, the faster the diffusion will occur

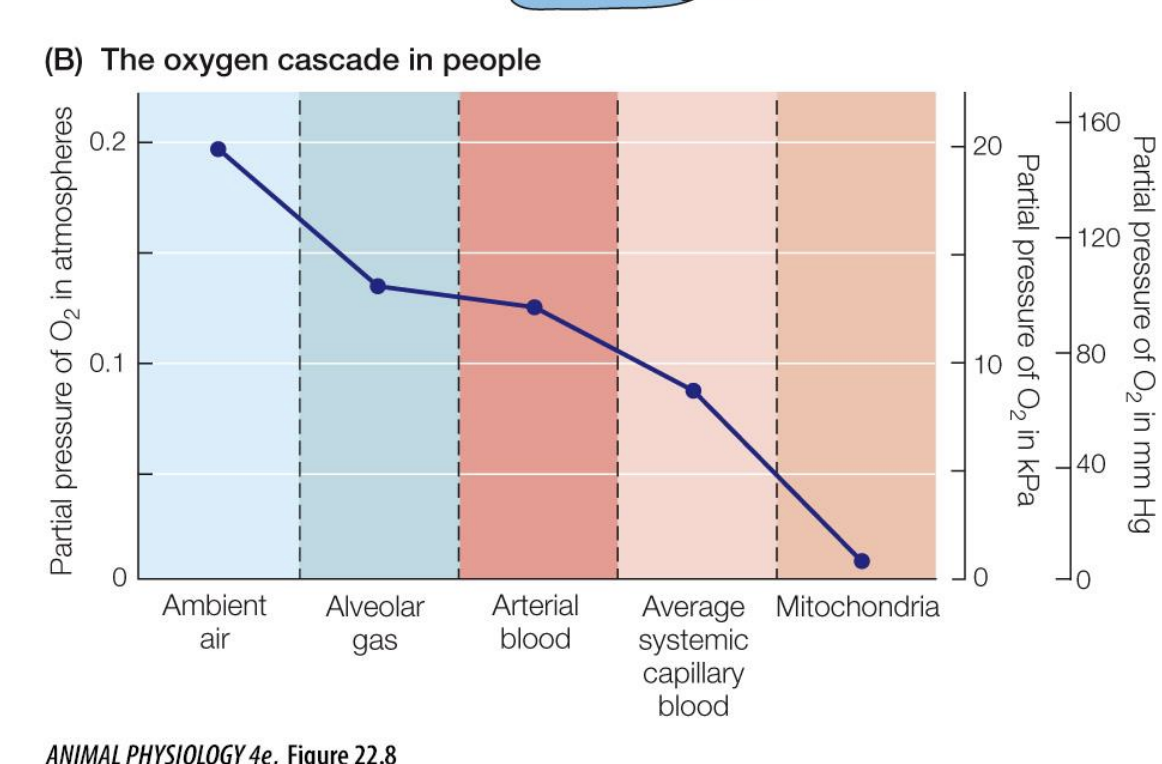
because there is no active transport of O2 in the body, what is the gradient like?
it is always passive, and the partial pressure of O2 will only go down as it travels from the air to the alveolar to the arterial blood to systemic capillaries to the target tissue