Unit 2 - V. Electron Configuration & Quantum Emission
1/9
Earn XP
Description and Tags
Don't use shuffle mode.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
E-1 configuration is orbital diagram but quicker, leaving out:
electron spin
specific orbitals in sublevel
paired vs. unpaired
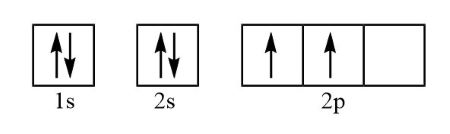
orbital diagram
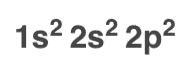
electron configuration
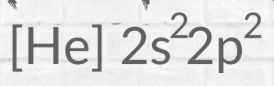
noble gas shorthand
helps us see valence electron quicker
Energy level relative to
distance from nucleus
As n increases, ____ but ____.
distance from nucleus increases, but energy gap gets smaller
Since electrons are packets of energy,
even light can donate energy to an electron
If an electron gets enough energy from an outside source so that it has enough energy to exist in a different orbital,
it will teleport there! (excited state)
This gained energy is lost quickly, so
the electron will fall back down to its ground state
EM radiation is given off
Electromagnetic radiation
packet of energy called a photon
color is influenced depending on energy of photon