Topic B Flaschards
1/57
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
58 Terms
three states of matter
(B.1: Thermal Energy Transfers)
solid, liquid, gas
internal energy
(B.1: Thermal Energy Transfers)
the sum of the total random kinetic energy and the total intermolecular potential energy of its particles.
specific heat capacity
(B.1: Thermal Energy Transfers)
the amount of heat energy required to change the temperature of 1 kg of mass by 1 C or 1 K.
specific latent heat
(B.1: Thermal Energy Transfers)
Measurement of how much energy it will take to change 1 kg of material from one phase to another.
changing kinetic energy affects...
(B.1: Thermal Energy Transfers)
temperature
changing potential energy affects...
(B.1: Thermal Energy Transfers)
the bonds between the particles.
fusion vs vaporisation latent heat
(B.1: Thermal Energy Transfers)
fusion: solid-liquid
vapourisation: liquid-gas
from solid to gas...
(B.1: Thermal Energy Transfers)
- kinetic energy of individual particles increases, meaning that temperature increases.
- intermolecular bonds become weaker
- higher potential energy
- thermal expansion
During a phase change...
(B.1: Thermal Energy Transfers)
the average potential energy of the particles changes. The average kinetic energy doesn't change- temperature doesn't change.
conduction
(B.1: Thermal Energy Transfers)
The direct transfer of heat from through particle-particle collisions and movement.
Objects must be touching.
Heat flows from high to low temperature areas due to particle collisions.
convection
(B.1: Thermal Energy Transfers)
energy transfer through the distribution and movement of a fluid (liquid or gas).
More heat= rise up from more dense to less dense are
Cools down= Goes back down to heat area from less dense to more dense area
radiation
(B.1: Thermal Energy Transfers)
energy transfer through electromagnetic radiation that each body emits.
Black body
(B.1: Thermal Energy Transfers)
Object that absorbs and emits all wavelengths of light from the electromagnetic spectrum. (eg. stars)
Luminosity
(B.1: Thermal Energy Transfers)
The total power emitted by a star in the form of electromagnetic radiation.
Thermal equilibrium
(B.1: Thermal Energy Transfers)
Equal the equations
Objects in thermal contact
Heat Lost = Heat Gained: In many problems, you'll need to use this principle to find final equilibrium temperatures when different materials are mixed or when heat is transferred.
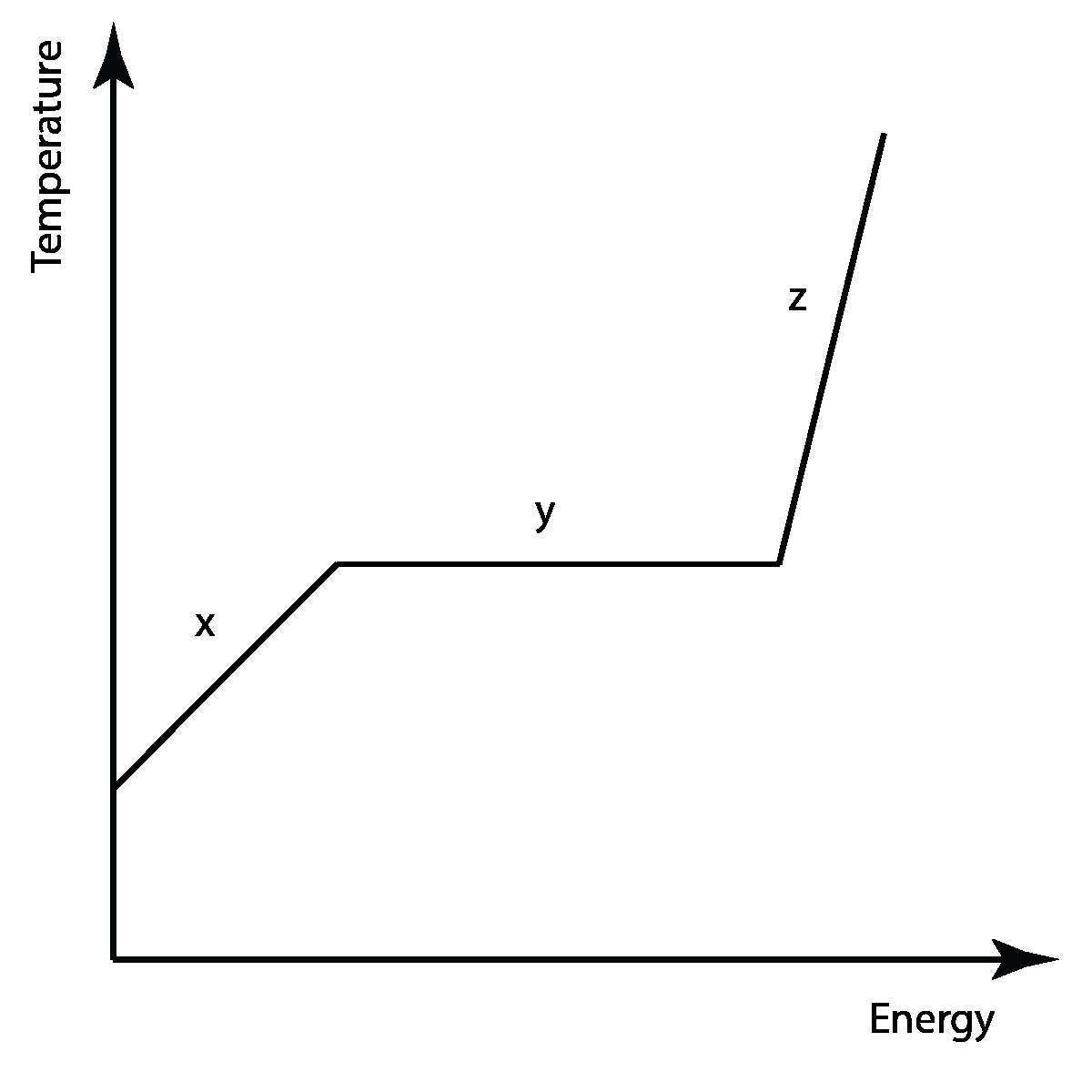
Interpret this graph
(B.1: Thermal Energy Transfers)
X: temp increasing
Y: Melting point/Boiling point reached. Phase is changing. Melting/vaporizing
Z: Phase 2 reached
eg.
X: Liquid
Y: Boiling point: Liquid to Gas
Z: Gas
Heat transfer rule
(B.1: Thermal Energy Transfers)
Heat lost by a hotter object: Tinitial- Tfinal
Heat gained by a cooler object: Tfinal- Tinitial
Ideal Gas
(B.3: Gas Laws)
A gas that follows the ideal gas law
No intermolecular bonds
All collisions must be perfectly elastic.
Particles are moving in random directions
Small identical molecules
Have more kinetic energy than potential energy
Conditions for ideal gas
(B.3: Gas Laws)
High temperatures: More Ek
Low pressure and density: Less interaction between particles, so less intermolecular forces
Pressure vs. Temperature at Constant Volume graph
(B.3: Gas Laws)
Linear
Volume vs. Temperature at constant pressure graph
(B.3: Gas Laws)
Linear
Pressure vs Volume at constant temperature
(B.3: Gas Laws)
Inverse nonlinear relationship (decreasing curve).
1 Law of thermodynamics
(B.4: Thermodynamics)
When energy is put into a gas by heating or working on it, its internal energy must increase.
Q= U + W
2nd Law of thermodynamics
(B.4: Thermodynamics)
In every process, the total entropy of an isolated system always increases
Celsius form: Thermal energy cannot spontaneously (without doing work) transfer from a region of lower temperature to a region of higher temperature
Kelvin form: When extracting energy from a heat reservoir, it is impossible to convert it all into work
Graphs of Constant pressure and volume
(B.4: Thermodynamics)
Can provide information about the work done and internal energy of the gas
Area= Work done
volume increases= work is done BY the gas
Volume decreases: arrow is reversed (work is done ON the gas)
Entropy
(B.4: Thermodynamics)
measure of how disordered a system is
The order of entropy for the different states of matter from most disordered to least is: gas > liquid > solid
Microstate
(B.4: Thermodynamics)
A microstate describes the number of states or possible arrangements of the particles in the system
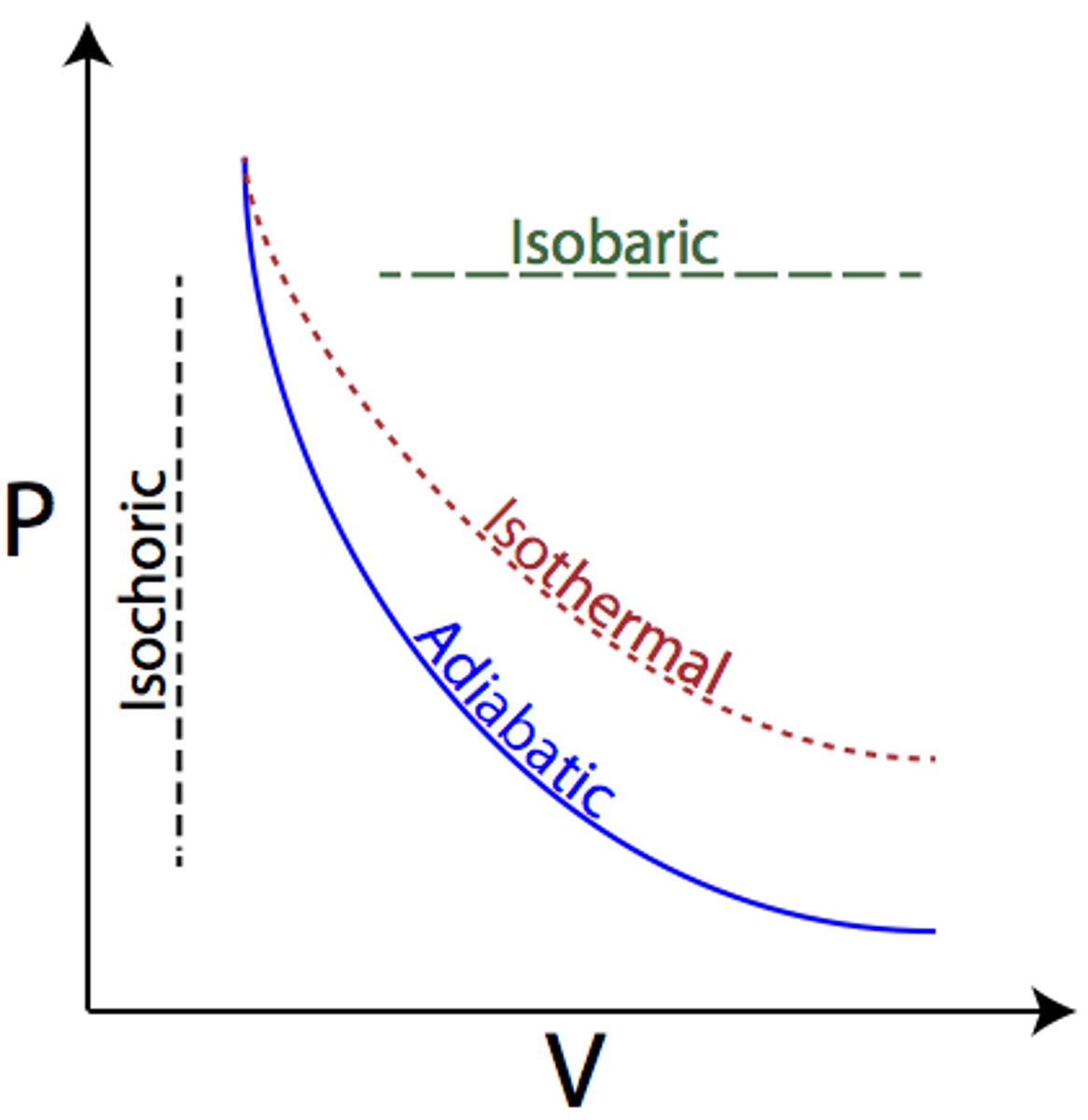
Four thermodynamic processes
(B.4: Thermodynamics)
Isovolumetric W=0
Constant Volume
Isobaric Δp=0
Constant pressure
Isothermal ΔU=0
Constant Temperature
Adiabatic ΔQ=0
A process where no heat is transferred into or out of the system
Isobaric
(B.4: Thermodynamics)
Occurs when gasses are allowed to expand or contract freely during a change in temperature (Volume changes but pressure stays the same)
Expansion: Heat gained= increase in internal energy + work done by gas
Compression: Heat lost= decrease in internal energy + work done on gas
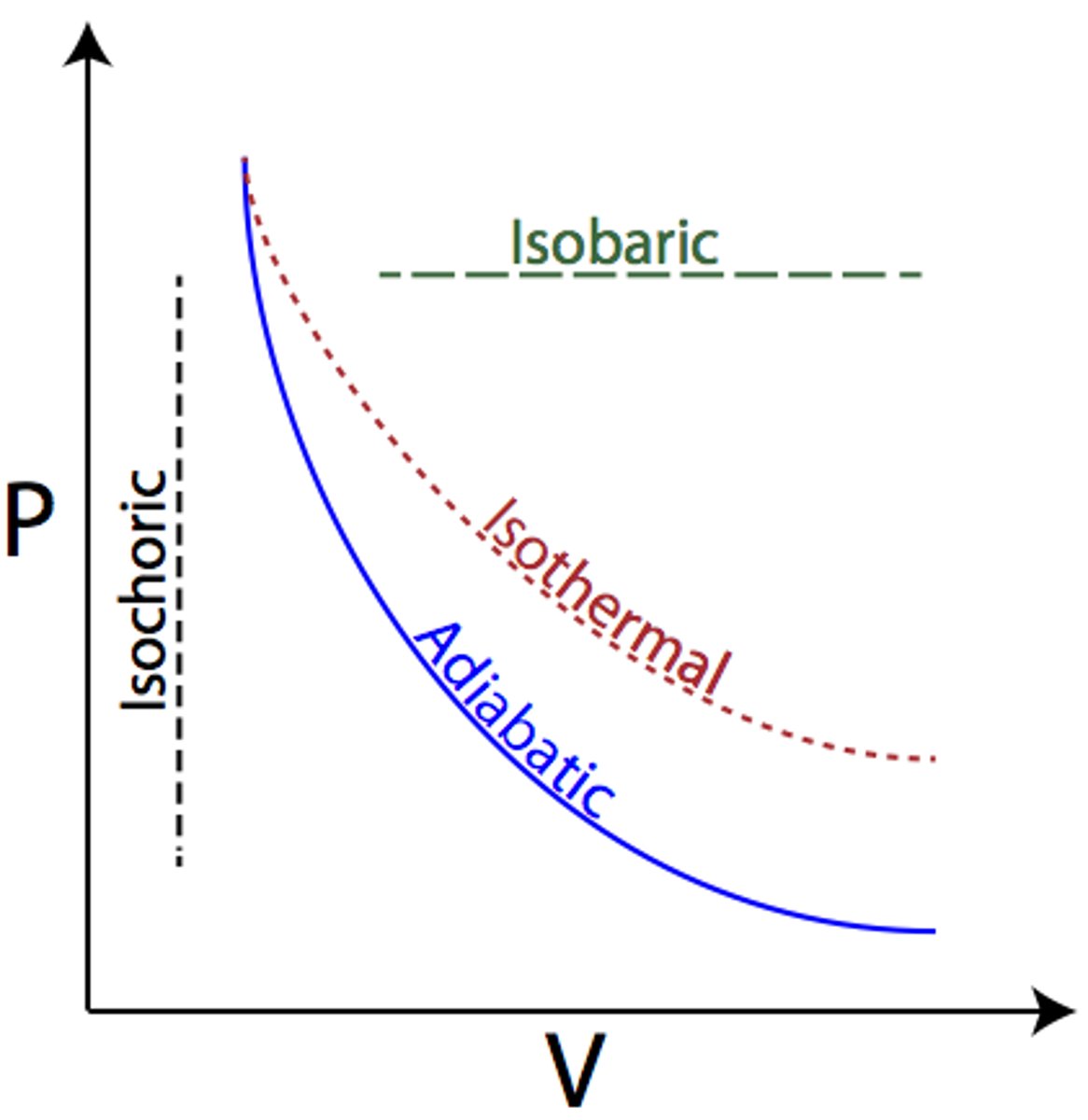
Isovolumetric
(B.4: Thermodynamics)
Q=ΔU
(Isochloric in graph)
Heat gained due to temperature rise
Heat lost due to temperature drop
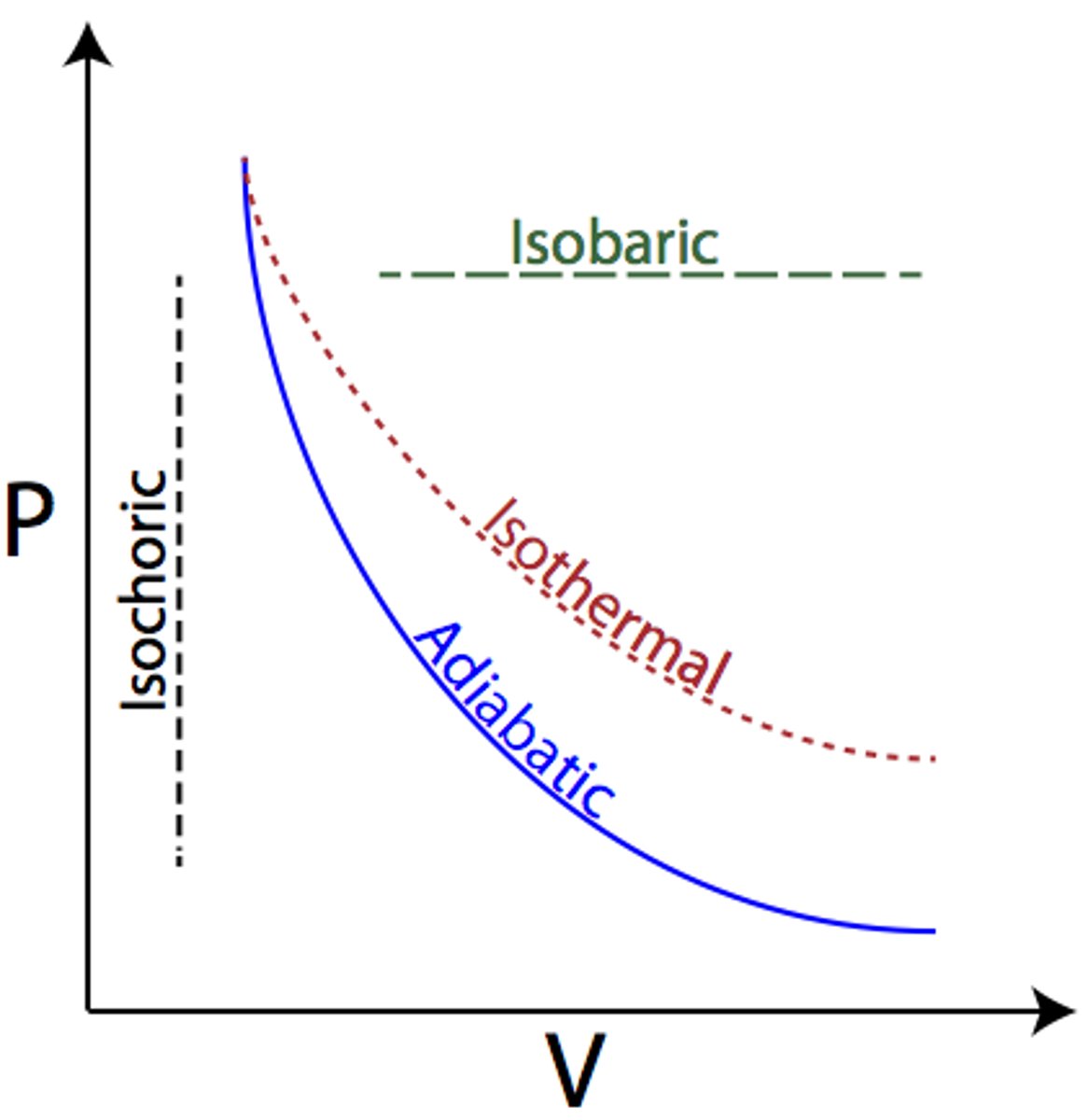
Adiabatic
(B.4: Thermodynamics)
W=-ΔU
Steeper graph line than Isothermal process.
Expansion: Pressure and temperature decrease w/ no heat gained or lost
Compression: Pressure and temperature increase w/ no heat gained or lost
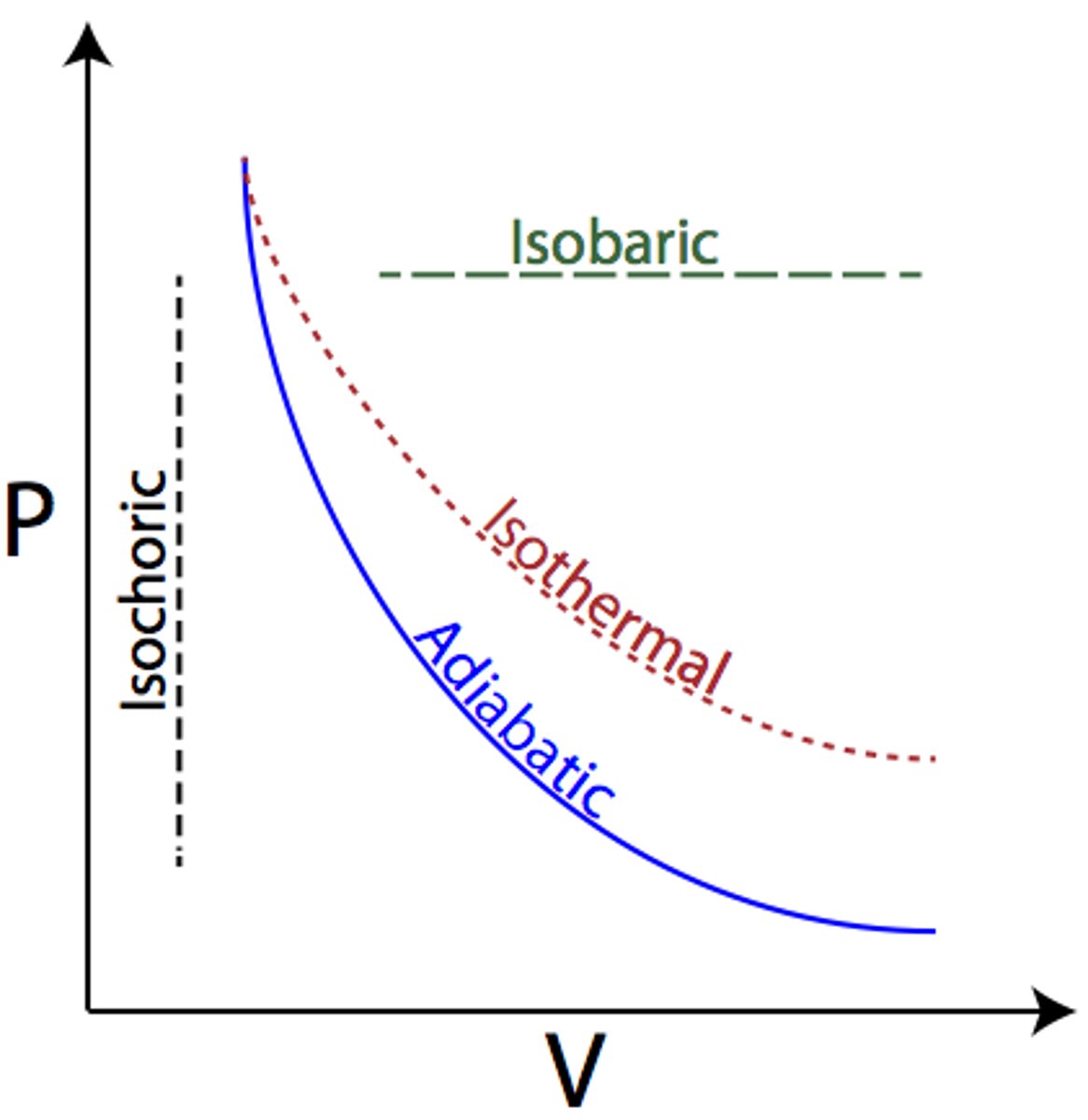
Isothermal
(B.4: Thermodynamics)
Q=W
Expansion: Heat gained= Work done by the gas
Compression: Heat lost= Work done on gas
Heat engine
(B.4: Thermodynamics)
Device that converts thermal energy into mechanical work
Work done= area under PV graph
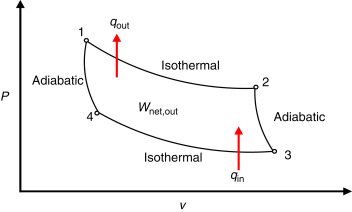
Heat engines in carnot cycles
(B.4: Thermodynamics)
Isothermal expansion: gas absorbs heat at constant temp (TH)
Adiabatic expansion: Cools from TH to Tc
Isothermal: Gas releases heat at constant temp (TC)
Adiabatic compression: Increasing gas temp from Tc to TH
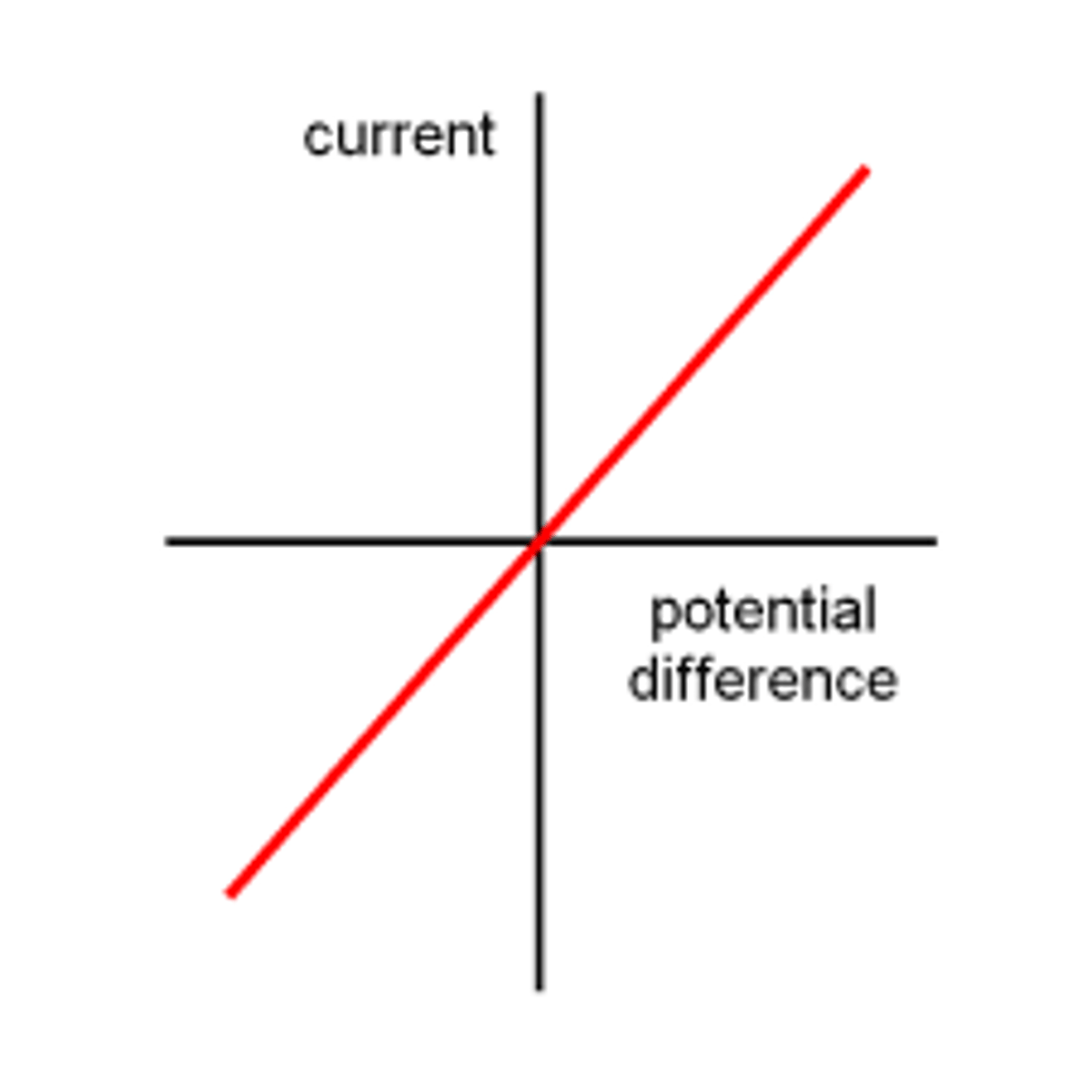
Ohm's Law
(B.5: Current and Circuits)
For a component at a constant temperature, the current through it is proportional to the potential difference across it
V∝I
Ohmic Components
(B.5: Current and Circuits)
A component is said to obey Ohm's Law (an ohmic component) if its current vs. voltage graph is a straight line through the origin. eg. a fixed resistor.
Conductors
(B.5: Current and Circuits)
materials that allow charge (usually electrons) to flow through them easily.
-often metals
Insulators
(B.5: Current and Circuits)
materials that do not allow charge to flow easily because they lack free charges.
Examples: Rubber, plastic, glass, wood
Causes of electric resistance
(B.5: Current and Circuits)
collisions increase temperature due to increased kinetic energy
direction of an electric current
(B.5: Current and Circuits)
the direction in which positive charge would flow.
How do particle behaviors differ in solids, liquids, and gases?
(B.1: Thermal Energy Transfers)
Solids: particles vibrate in fixed positions.
Liquids: particles slide past each other.
Gases: particles move freely and randomly.
What determines the direction of heat transfer?
(B.1: Thermal Energy Transfers)
The temperature difference.
What does conservation of energy mean in climate systems?
(B.2: The Greenhouse Effect)
Energy in = Energy out
Energy absorbed= Energy emitted
What is emissivity?
(B.2: The Greenhouse Effect)
The proportion of radiation emitted by a gray body compared to a black body.
What is albedo?
(B.2: The Greenhouse Effect)
The fraction of incoming energy that is reflected by a surface.
“What percentage of energy is reflected”
What affects Earth's albedo?
(B.2: The Greenhouse Effect)
Cloud cover, ice, land types, and latitude—it varies daily.
What is the solar constant (S)?
(B.2: The Greenhouse Effect)
The amount of solar energy per second per m² received at Earth's upper atmosphere
-Perpendicular to the Sun's rays when earth is at average distance from the sun
Why is the average solar power over Earth's surface S/4?
(B.2: The Greenhouse Effect)
Because only a circular cross-section of Earth receives sunlight at once, but energy is spread over the whole spherical surface.
Average solar radiation
(B.2: The Greenhouse Effect)
340 W/m2
Greenhouse effect
(B.2: The Greenhouse Effect)
Natural process where the earth's atmopshere traps some of the Sun's energy, keeping the planet warm enough to support life.
Positive feedback loop (global warming)
(B.2: The Greenhouse Effect)
A positive feedback effect will increase the rate of temperature increase
Earth heats up-> snow melts reducing albedo -> more radiation is absorbed by water -> temps continue increasing, snow continues melting
Negative feedback loop (global warming)
(B.2: The Greenhouse Effect)
A negative feedback effect will decrease the rate of temperature increase
Watervapour-> clouds-> reflects more
Enhanced greenhouse effect
(B.2: The Greenhouse Effect)
Extra warming due to human activities increasing greenhouse gas levels beyond natural levels.
Outline the greenhouse effect
(B.2: The Greenhouse Effect)
1. Shortwave solar radiation reaches the earth
2. Some of this is reflected back into space or absorbed by the Earth
3. The earth then emits infrared raditation back into space: which has longer wavelengths than solar radiation
4. Some of this infrared radiation is absorbed by greenhouse gases which re-emit this energy in all directions
5. Some of this energy escapes into space but much of it is directed towards earth, "trapping heat" and re-warming the planet
When is a gas well-approximated by the ideal gas model?
(B.3: Gas Laws)
Low pressure, high temperature, and low density.
What sets the maximum efficiency of a heat engine?
(B.4: Thermodynamics)
The carnot cycle
Lost Volts
(B.5: Current and Circuits)
The energy wasted per coulomb overcoming the internal resistance of a power source
Voltage vs. Current graph
(B.5: Current and Circuits)
Gradient: Internal resistance of the cell
y-intcept: Terminal voltage (emf)