Topic 1 - Atomic and Molecular Orbitals
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What is an atomic orbital?
A wave function that defines a region with a high probability of finding an electron.
It can be described by specifying size, shape and directional properties.
A density plot can show orbitals.
What does a conventional 1s orbital look like?
Spherical, with no nodes.
What is a node?
An area where the probability of finding an electron is 0.
What does a conventional 2s orbital look like?
Spherical, with a spherical node.
It is higher energy than 1s.
What does a conventional 2p orbital look like?
Dumbbell-shaped, not spherical and has a 2D nodal plane.
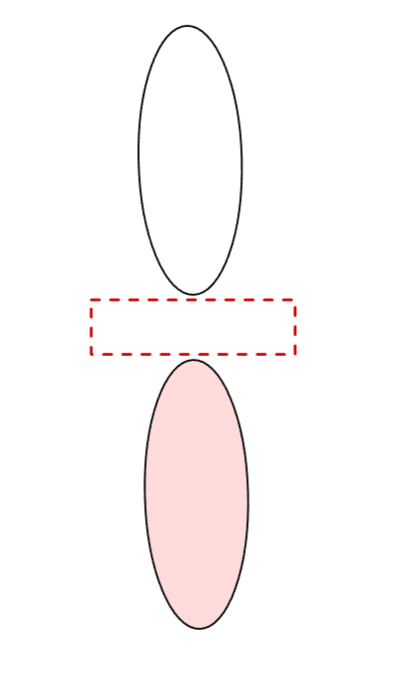
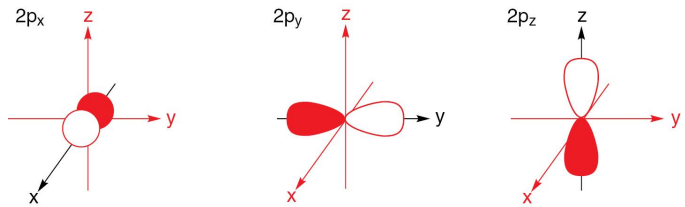
What cartesian axis coordinates do the different p-orbitals occupy?
The px orbital occupies the x-axis, the py orbital occupies the y-axis, and the pz orbital occupies the z-axis.
What is Hund’s Rule?
Electrons will fill degenerate (same energy) orbitals singly before pairing up. This minimizes electron-electron repulsion and maximizes total spin.
Why is the energy of the 1s orbital of helium lower than that of hydrogen?
Helium has more protons, so has a greater nuclear attraction to electrons. This means that the electrons are lower in energy.
This is the same for lithium and beryllium.
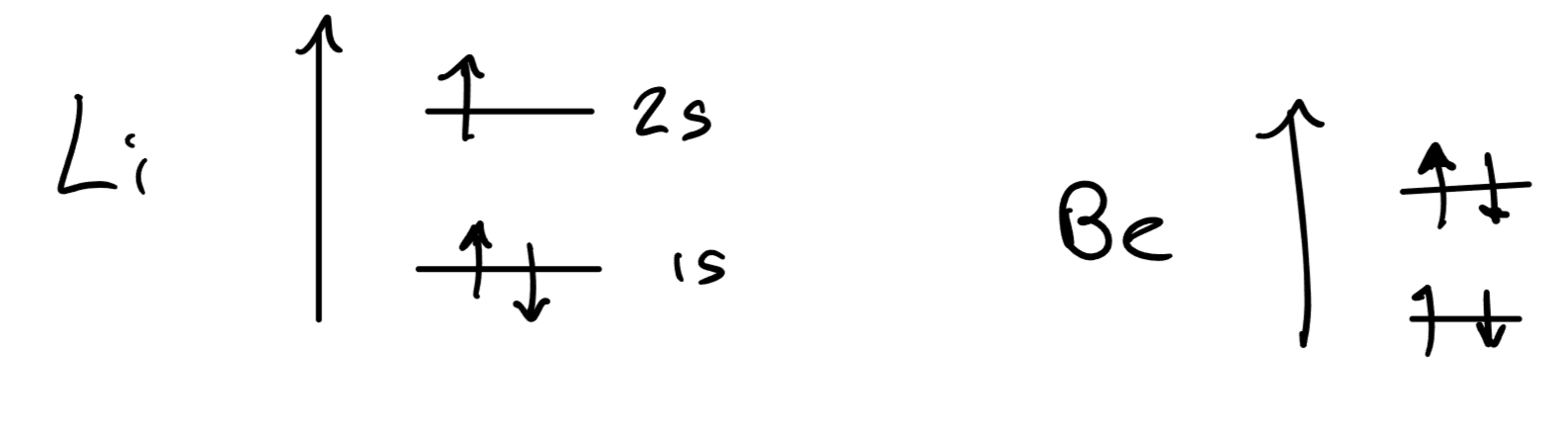
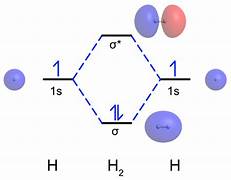
What is a molecular orbital?
Molecular orbitals are combinations of atomic orbitals.
Atomic Orbitals (AOs) can be combined in or out of phase.
What is the subtractive combination?
It is out of phase - a destructive interaction, producing an antibonding molecular orbital (MO*).
What is the additive combination?
It is in phase - a constructive interaction, producing a bonding molecular orbital (MO).
What is the molecular orbital diagram for H2?
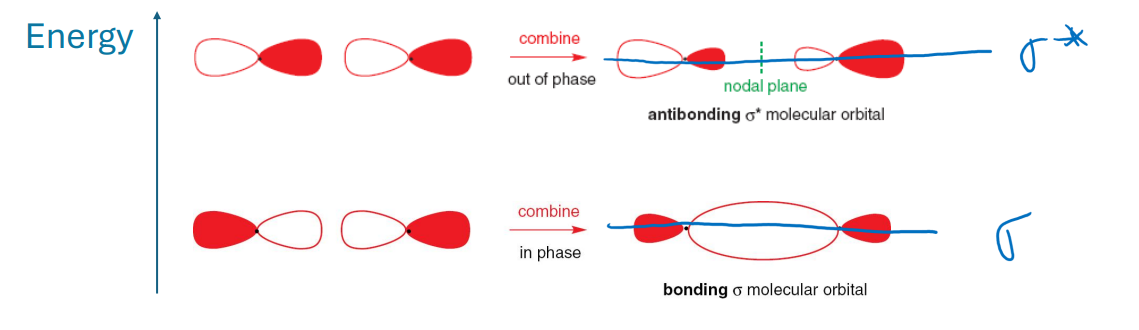
What are σ bonds?
Bonds made of σ and σ* orbitals.
σ bonding is often described as ‘end-end overlap‘.
σ bonding orbitals and σ* antibonding orbitals are symmetrical by rotation around the bond axis.
What are the prerequisites to forming molecular orbitals?
Atomic Orbitals must be similar energy.
→ If two atomic orbitals are different in energy, an electron transfer occurs, producing an ionic salt.
Atomic Orbitals must be similar size.
→ For the best overlap, orbitals should be the same size.
Atomic Orbitals must have appropriate symmetry.
→ This is required for combination.
How can p-orbitals form?
End on, and edge on overlap.
What is end-on overlap in p-orbitals?
A type of orbital overlap where two p-orbitals align along the axis of the bond, allowing for effective interaction and bonding.
It is symmetrical about the internuclear axis.
This interaction is responsible for the production of σ bonds.
What is edge-on overlap in p-orbitals?
A type of orbital overlap where two p-orbitals align perpendicular to the axis of the bond, allowing for side-on interaction.
It is not symmetrical about the internuclear axis.
This results in the formation of π bonds.
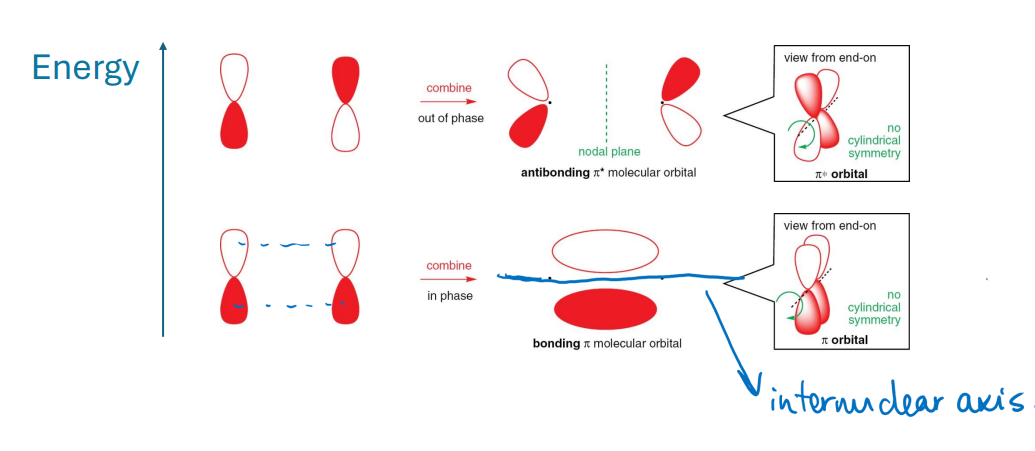
What is the difference between σ and π bonds?
σ bonds are formed through end-on overlap, while π bonds are formed through edge-on overlap of p-orbitals.
σ bonds have better overlap, and are lower in energy.