Avian Influenza and Poultry Notifiable Disease
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
What is a notifiable disease?
A notifiable disease is one that must be reported to the Animal and Plant Health Agency (APHA)- as soon as there are any suspicion that it may be present
It can be endemic in the UK, such as bovine TB or exotic e.g. food and mouth disease
Why is a disease “made” notifiable?
Three main reasons:
International trade
Many notifiable diseases will result in trade restrictions. World Organisation for Animal Health (WOAH) maintains a list of countries free of certain diseases, including avian influenza
Animal health and welfare
Highly infectious and destructive/ debilitating diseases with significant economic impact
Public health
Some notifiable diseases are potentially severe zoonoses
What happens if a notifiable disease if suspected?
*Compensation- compensated for alive animals only, animals that have already died of disease don’t get compensated for
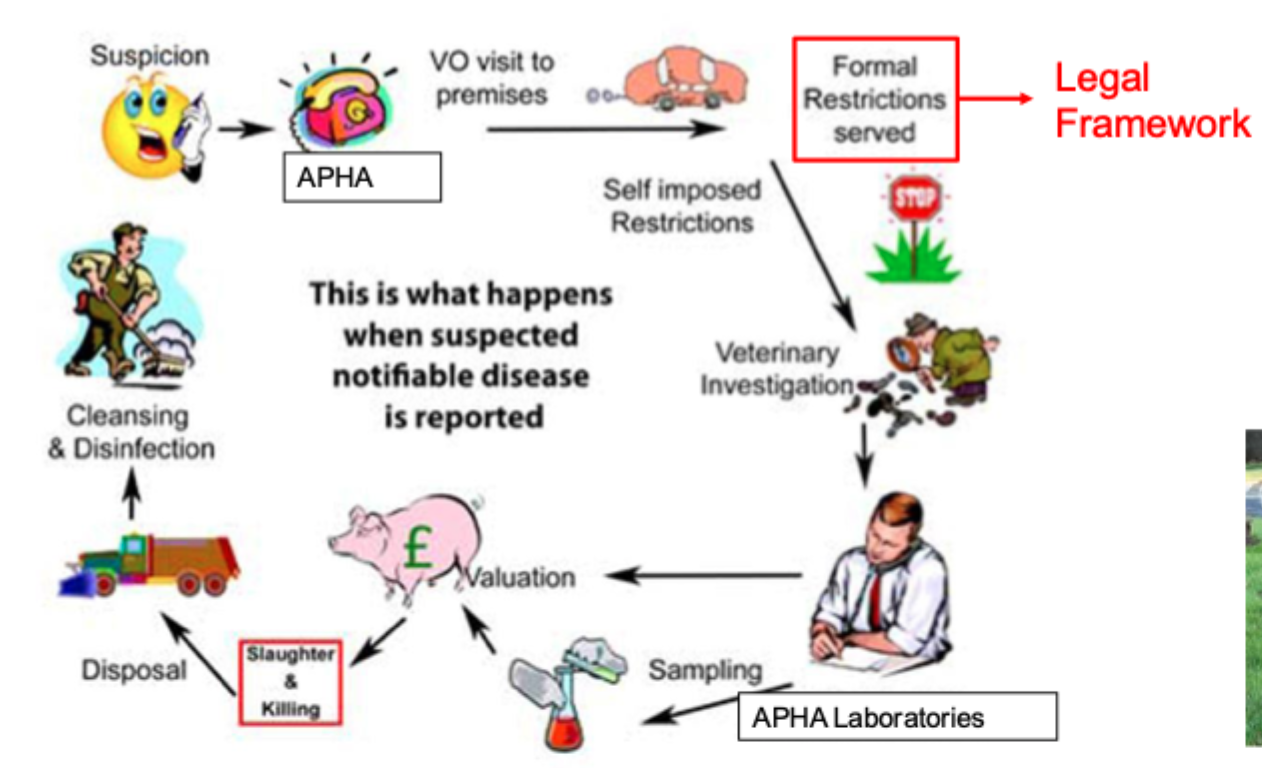
Notifiable avian disease
Avian influenza virus
Newcastle disease virus
*Can affect: chickens, ducks, turkeys
Avian Influenza
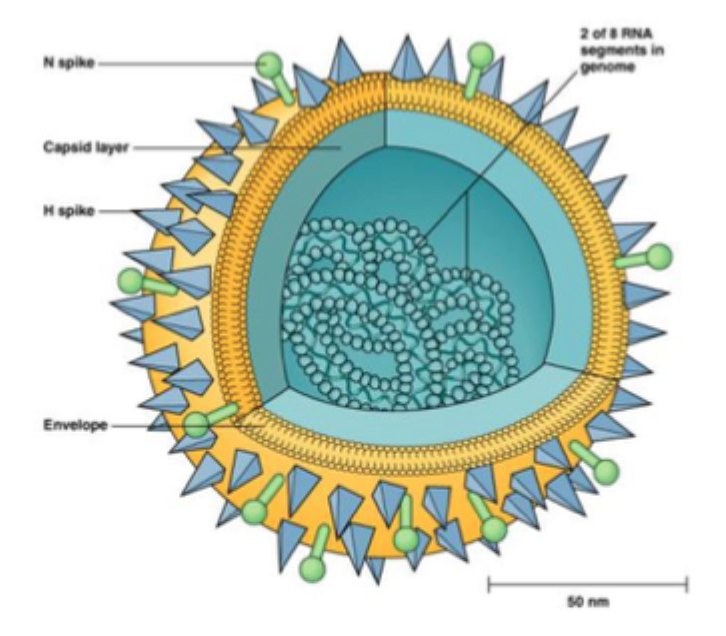
Orthomyxoviridae (-ve sense, ssRNA virus)
Influenza virus A
Influenza A variants can be named according to host species (e.g. ‘bird flu’, ‘swine flu’)
Standard naming: Influenza A/ turkey/ Turkey/ 1/05 (H5N1)
(Strain/ species/ country/ ??/ year)
May also be named according to pathogenicity, esp. in poultry e.g. low pathogenic AI, highly pathogenic AI
Very prone to genetic CHANGE (RNA viruses- no ability to proofread so change in occurs a lot)
Emergence of new subtypes based on point mutations and genome reassortment
Influenza A virus
Causes severe respiratory disease in humans and other animals
Epidemic (seasonal) or pandemic outbreak in humans
Classified on basis of viral surface proteins:
Haemagglutinin (HA or H)
Neuraminidase (NA or N)
Antibodies to HA are mostly responsible for virus neutralisation (NA less so)
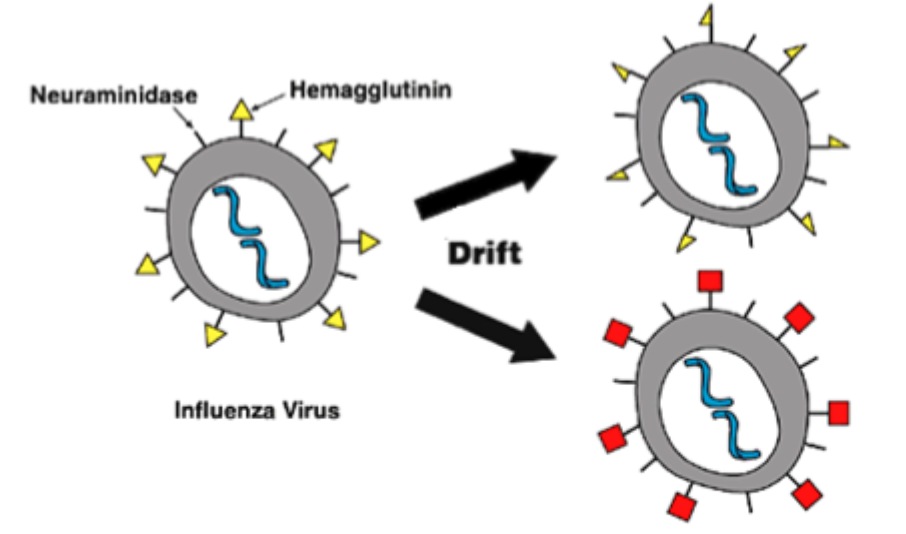
Influenza A virus evolution- Antigenic drift
RNA- dependent RNA polymerase that copies the viral genome makes an error roughly 1/ 10,000 nucleotides
10kb is the approximate length of the influenza RNA genome
This genetic change leads to gradual change in encoded proteins and so called “antigenic drift”
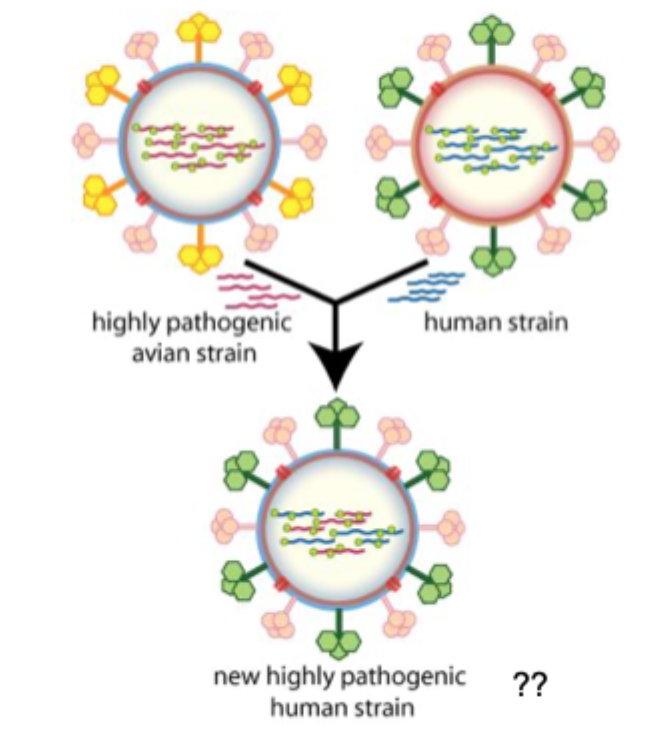
Influenza A virus evolution- Antigenic shift
Segmented genome (8 separate segments of vRNA)
Allows mixing or reassortment of vRNAs if more than 1 type of influenza virus infects a single cell. Leads to sudden changes in virus genome which can change resulting proteins dramatically- antigenic shift.
Pandemic H1N1 2009 (swine flu 2009) virus is a previous triple reassortment of bird, pig and human flu viruses further combined with an Eurasian pig flu virus.
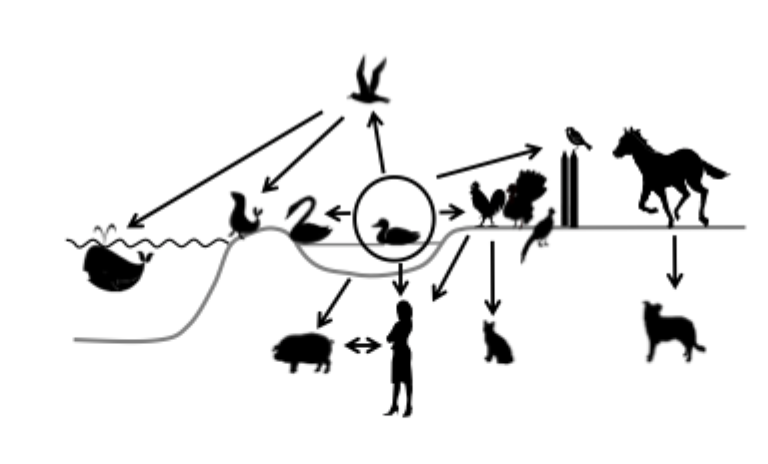
Avian influenza virus epidemiology
Aquatic birds especially ducks reservoir for most avian influenza viruses
Migratory waterfowl can spread virus (main cause)
Avian influenza isolates usually species-specific (enabling virus to change and adapt between species)
Replicates inside the intestinal tracts of birds then transmission is by the faecal oral route + aerosol
Can also cause respiratory infection
Severity of avian influenza
Pathogenicity varies between subtypes from low morbidity with low pathogenic viruses to 100% mortality with highly pathogenic viruses (HPAI)
HPAI H5N1 virus causing current outbreaks in UK
Highly pathogenic AI (HPAI)
Sudden Death (upto 100% mortality in 72 hours)
Reduced feed consumption
Cessation of flock vocalisation
Drop in production (egg or live weight gain)
Depression
Cough
Cyanosis
Diarrhoea
Nervous signs- off thier feet
*off purple colour in wottle
Highly Pathogenic AI (HPAI)
What does it take to make a highly pathogenic AI virus?
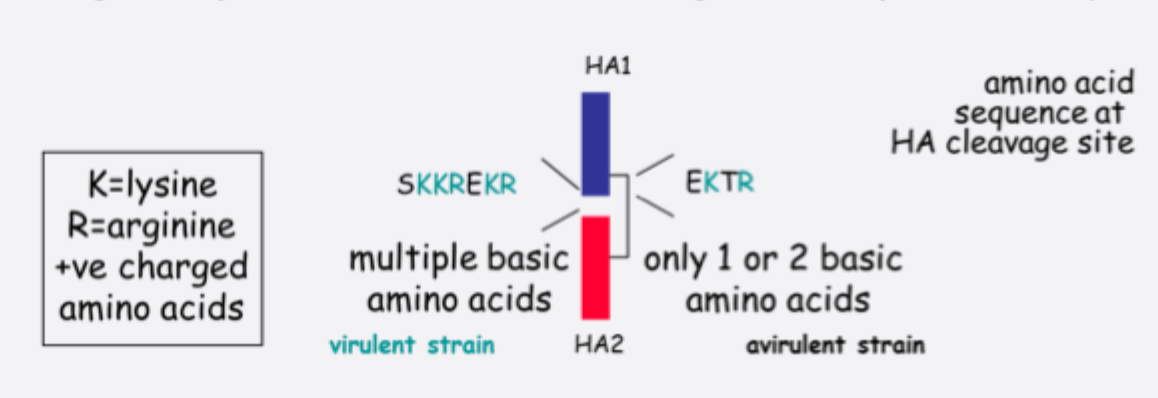
Highly pathogenic strains of AI are H5 or H7
Pathogenicity associated with cleavage of HA (cf. NDV F protein)
*only able to be cleaved in gut or respiratory tract- EKTR- low pathogenic RKKREKR- can be cleaved anywhere- highly pathogenic
Can spread through whole body hence causing more problems
International definition of notifiable avian influenza
Avian influenza due to highly pathogenic H5 or H7 viruses
Avian influenza with an intravenous pathogenicity index (0-3) in 6 week old chickens greater than 1.2 or 75% mortality over 10 days.
Also: avian influenza due to low pathogenic H5 or H7 viruses
A notifiable avian influenza outbreak requires immediate implementation of control measures in most countries through national legislation.
*lots of low pathogenic diseases circulating, ability to become highly pathogenic- H5 or H7
On suspicion of HPAI
APHA notified by vet, farmer ect
Farmer must not move anything (live birds, manure etc) off premises
Birds cannot be brought onto premises
APHA vet examines birds, records etc (may rule out AI)
Premises declared a “suspect premises”
VI serves notice placing controls on movement of animals, eggs, vehicles etc.
Isolation of poultry from wild birds within buildings (free- range birds)
Deployment of disinfection facilities at entrance to premises and bird housing
Warning signs erected
Diagnostic testing for NDV or AIV
When is testing done?
2 main scenarios:
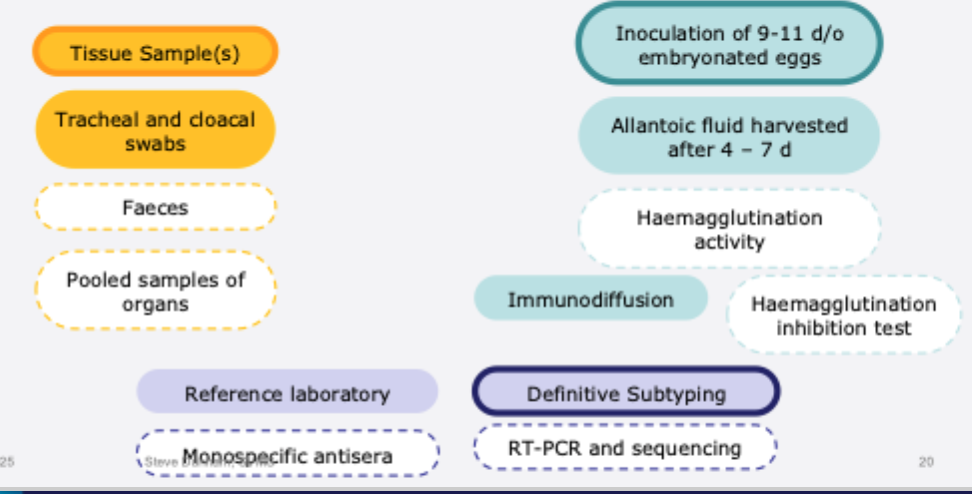
1- Outbreak of ND/ AIV is suspected (notifiable disease investigation)
Samples may include swabs, tissue samples, cadavers
Egg inoculation to grow and isolate virus
RT- PCR diagnosis for virulence determinants (e.g. cleavage site)
2- For exclusion of ND or AIV as a possible diagnosis
Cloacal and oropharyngeal swabs collected from ~20 birds
Samples pooled and tested by rRT- PCR for avian influenza or paramyxovirus genes
Diagnostic tests for AI
Once disease is confirmed
Actions are initiated through the implementation of government contingency plans
Notification of infected status to WOAH (effects UK disease free status)
Actions at infected premises
Disease control zone
AI prevention zones- enhances biosecurity +/ - mandatory housing
Actions at infected premises
Culling- all poultry and any captive birds
Disposal- all carcases, eggs and any other contaminated material must be disposed of
Initial cleaning and disinfection (C&D) will be carried out by APHA
Health measures for exposed workers dependent on risk
Undertake secondary C&D and restock after further 21d or
Cease production and maintain biosecutiy for 12 months- suggestion that ~50% of flocks may do this!
Control zones around infected premises
HPAI (except H5N1 in poultry)
Protection zone (PZ) 3km 21d
Surveillance zone (SZ) 10km 31d
HPAI H5N1 in poultry
Protection zone (PZ) 3km in 21d
Highly restricted in terms of movement and biosecurity
Surveillance zone (SZ) 10km 30d
Similar but meat/ carcass can be removed
Optional restriction zone (RZ) 30d
No restrictions on movement of litter
Culling
Cull birds as soon as possible in a way that minimises risk of onward disease spread
Welfare of birds is subject to legal controls
Whole house gassing is preferred
Ventilation shutdown a last resort where there is a threat to public health and only permitted in England
Culling- whole house gassing (WHG)
WHG with CO2, where birds are killed in their production housing, is the preferred option. But not all poultry buildings are suitable for WHG due inadequate sealing.
Or the use of high expansion water- based foam to deliver an anoxic gas (nitrogen) intro poultry sheds.
*needs to be airtight for gassing, foam hard to clear up
Culling- containerised gassing unit
Birds are placed inside a standard industry transport module and loaded into a solid rectangular gas- tight steel container with a hinger door with 2 inlets for gas and 2 outlets through which air is displaced. The gas used is 80% argon and 20% CO2, this is commercially available throughout the world as it is used for welding.
When 2 CGUs are operated in tandem and operated to thier full potential, system is capable of killing 4-5000 birds per hour
Compensation
For poultry or other captive birds not diseased at the time of killing, compensation is payable at the value of the birds immediately before killing.
For poultry or other captive birds dead or diseased at the time of killing, no compensation is payable.
Amount of compensation determined from “rate tables”, depending on age of birds, broiler vs layer etc
Does not account for “consequential losses”
*Doesn’t take into account cleaning and disinfecting costs
*Doesn’t take into account cost of time not in production
Current UK situation
Ongoing outbreaks of H5N1 in poultry
Large number of cases of HPAI H5N1 in England since the H5N1 outbreak started in October 2021
An avian influenza prevention zone (AIPZ) including mandatory biosecurity measures is in force across England, Wales and Scotland
Some parts of England also require mandatory housing of birds
ALL poultry keepers must be registered with APHA
Rules in AIPZ
Strict biosecurity and hygiene including:
Keep ducks and geese separate from other birds
Prevent contact with wild birds and rodents
Clean housing, equipment, vehicles and footwear
Keep records of birds movements, death and eggs
Additional rules if you keep more than 500 birds
Housing required in some regions
Impact on wildlife
Large number of infected species including geese, ducks, seabirds, birds of prey- many deaths in previous years
Populations of rare and endangered species at risk
Vaccination against avian influenza
Why not usein UK?
Vaccination prohibited in ‘slaughter policy’ countries because of difficulty establishing freedom from infection
Vaccines effective against one subtype may not be effective against another
Live vaccines are not used due to risk of reversion to virulence
Vaccinated asymptomatic birds may still shed viruses
Used widely in some asian and South American countries
Permitted in UK for some birds in zoological collections
Influenza at the human- animal interface
Influenza viruses circulating in animals pose threats to human health e.g. avian influenza virus H5N1 and H9N2
Contact with infected animals at work
Live animal markets, close contact in “family situations”
Handling or slaughter of infected animals, or work with raw meat and by- products from infected animals
Contamination from faeces, blood etc.
Avian Influenza summary
Caused by influenza A viruses
Virus change through mutation and genome reassortment make effective vaccination very difficult
Strains are usually species specific but some risk of humans zoonosis and potential for human pandemic
Spread of AI facilitated by migration of wild birds
UK has control zone and slaughter policy
Newcastle disease virus
Avian paramyxovirus-1: affects chickens, ducks, pheasants, geese, turkeys
Shed in all excretions & secretions → aerosols
Virus stable for weeks on carcasses → mechanical transfer
Highly contagious
Affects all birds of all ages
Mortality rate variable: 50-100%
Common in wild birds- inapparent infection
Can cause mild conjunctivitis/ FLI in humans
*FLI is flu like illness
Newcastle disease virus
NDV strains vary in virulence and tropism:
lentogenic- mild inapparent infection
mesogenic- mild respiratory disease, some deaths in young birds
neurotrophic velogenic- acute, severe, fatal with respiratory and nervous signs
viscerotropic velogenic- severe, fatal with haemorrhagic lesions, respiratory disease
Determined by F glycoprotein
Newcastle disease virus
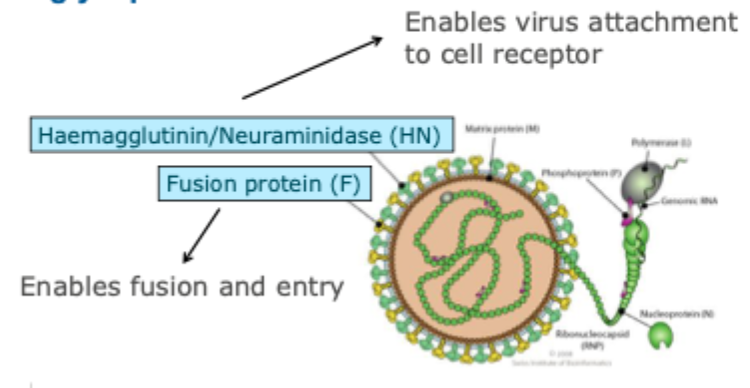
Membrane glycoproteins
HN- allows in and out
F- fusion
Newcastle disease virus
Fusion Protein
Synthesised as inactive precursor (F0)
Cleaved by host cell proteases to active forms (F1 and F2)- if not cleaved virus cannot enter!
Lentogenic strains are confined to GI and respiratory tracts where trypsin- like proteases are present
Velogenic stains can be cleaved in many tissues by ubiquitous (furin- like) proteases due to an altered cleaved site → contains multiple basic amino acids i.e. at least 3 Arg or Lys residues
Newcastle disease virus- previous UK outbreaks
1997- outbreak limited to 4 chicken flocks and 7 turkey flocks
2005- reared pheasant in Surrey*
2006- game birds in East Lothian (pigeon PMV- 1)
>10,000 birds slaughtered
Newcastle disease virus
Clinical findings
Vary depending on virulence of the virus
Depression, diarrhoea, oedema of the head, nervous signs, prostration, egg drop/ malformation
Watery yellow/ green diarrhoea
Shell malformation
Infected chickens with nervous sings
Haemorrhagic lesions
Newcastle disease virus- control
Biosecurity and other management techniques
Vaccination: most commercial flocks are vaccinated
live attenuated virus, inactivated or vectored vaccines
Notifiable disease: slaughter in the event of a severe outbreak with velogenic virus