TERM 3 BIOLOGY- Sem 2 24'
1/127
Earn XP
Description and Tags
C1.1.2, C1.1.3, C1.1.4, C1.1.10, C1.1.1, C1.1.5, C1.1.6,C1.1.7, C1.1.8, C1.1.9, C1.2.1, C1.2.2, C1.2.3, C1.2.4, C1.2.5, C1.2.6
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
128 Terms
metabolism describes the totality of
all enzyme catalysed reactions that occur within a living cell or organism
control over metabolism can be exerted through
enzyme specificity
two key metabolic reaction functions
provide source of energy for cellular processes and enable the synthesis and assimilation of new materials
anabolic reaction
metabolic reaction that builds up complex molecules from simpler ones
production of glucose by photosynthesis is
an anabolic reaction
catabolic reaction
metabolic reaction that breaks down complex molecules into simpler ones
oxidation of substrates in cell respiration is
a catabolic reaction
an enzyme is a globular protein which
acts as a biological catalyst by speeding up the rate of a chemical reaction
enzymes are not changed or consumed by
the reactions they catalyse and can be reused
enzymes are named after the substrate they react with meaning
they end with the suffix ase
lipids are broken down by the enzyme
lipase
proteins are digested by
proteases
the active site
region on the surface of the enzyme where the substrate binds to
interactions between active sites and animo acids
ensures that the overall shape and chemical properties complement the substrate
activation energy
every chemical reaction requires a certain amount of energy
enzymes speed up the rate of a biochemical reaction by
lowering the activation energy
less energy is required to convert
substrate into product with enzymes speeding up the reaction
exergonic
if reactants contain more energy than products, free energy is released into the system
exergonic reactions are usually
catabolic as energy is released from broken bonds
endergonic
reactants contain less energy than the products, free energy is lost to the system
endergonic reactions are usually
anabolic as energy is required to synthesise bonds
factors that affect enzymatic reactions
temperature, ph and substrate concentration
catalyst
a substance that allows a reaction to proceed at a faster rate or under different conditions
enzymes
biological catalysts that are not consumed by the specific reaction
enzymes allow chemical reactions to proceed within
a biologically relevant passage of time and biologically appropriate temperatures
without enzymes food would be unable to be chemically digested
within the period of transit through the digestive tract
without enzymes chemical reactions would
require higher temperatures which could denature components
enzyme catalysis requires that the substrate be brought into
close physical proximity with the active site
when a substrate binds to the enzymes active site
an enzyme-substrate complex is formed
enzymes catalyse the conversion of substrate into product
creating an enzyme-product complex
enzyme and product dissociate as
the enzyme was no consumed
induced fit model
enzyme’s active site is not a complete fit for the substrate
the active site will undergo a
conformational change when exposed to a substrate to improve binding
enzyme reactions occur in
aqueous solutions
brownian motion
substrate and enzyme moving randomly
sometimes, enzymes maybe be fixed in position
serving to localise reactions to particular sites
for enzymatic reaction to occur
substrate and enzyme must physically collide in correct orientation
rate of enzyme catalysis can be increased by improving
the frequency of collisions
two ways to increase frequency of collisions in enzyme catalysis
increasing molecular motion of particles, increasing concentration of particles
all enzymes have an indentation or cavity to
which the substrate can bind with high specificity
shape and chemical properties of the active site are highly
dependent on the three dimensional shape of the enzyme
enzyme structure can be modified by
high temperatures and extreme ph
high temperatures and extreme ph in enzyme structure can
disrupt chemical bonds, necessary to maintain shape and chemical properties
denaturation
change to the structure of the active site
denaturation will negatively affect
the enzyme’s capacity to bind to the substrate
inhibitors may also reduce
enzyme-substrate interactions by altering the shape of an active site
low temperatures reduce thermal energy
slowing enzyme-catalysed reactions
more kinetic energy means
more frequent enzyme-substrate collisions
excessive heat
destabilises enzymes, breaking hydrogen bonds
ph alters enzyme
charge, solubility and shape
enzymes work best at an optimal ph
outside of this activity decreases
more substrate
increases enzyme activity
high substrate concentration leads to
more collisions and reactions
beyond a certain point activity
plateaus as all enzymes are occupied
three key decisions to be made when designing an experiment to test the effect of factors affecting enzyme activity
specific enzyme/substrate reactions, experimental factor to manipulate, how to measure enzyme
rate of an enzyme-catalysed reaction can be calculates and plotted through
measure time take of consumed substrate, reaction rate is inverse of time take
reaction rate calculation
1/ time taken (s)
ATP is a molecule that
functions to distribute energy within cells
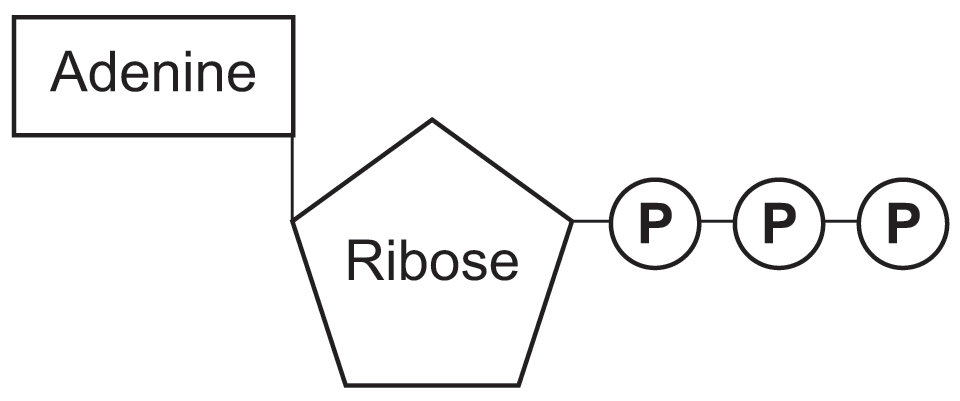
ATP is a ribonucleotide consisting of
an adenine base and three phosphate groups attached to the central ribose sugar
one molecule of ATP contains three covalently linked
phosphate groups which store potential energy in their bonds
when ATP is hydrolysed to release the outermost phosphate
energy store in the phosphate is released to be used by the cell
presence of adenine and ribose provides
additional sites for attachment to enzymes allowing ATP to fuel enzymatic activities
structure of ATP
there are a wide range of biochemical processes
that require use of ATP as an energy source
biosynthesis
assembly of organic polymers that requires ATP hydrolysis
anabolic reactions use ATP to construct
complex molecules from simpler subunits
with active transport ATP is required
to move material against a concentration gradient
nerves utilise ATP
to establish a resting potential prior to generating a nervous impulse
vesicular transport requires ATP
to break and reform membranes
movement of cell components or the whole cell is
dependent on ATP
chromosomes are segregated during mitosis and meiosis in an
energy-dependent process
contraction of muscle cells involves
the use of energy
coenzymes
non-protein organic compounds that facilitate enzyme reactions by cycling between a loaded and unloaded form
ATP is a loaded coenzyme that
transfers chemical energy to enzymes and enables the activation energy threshold to be reaches
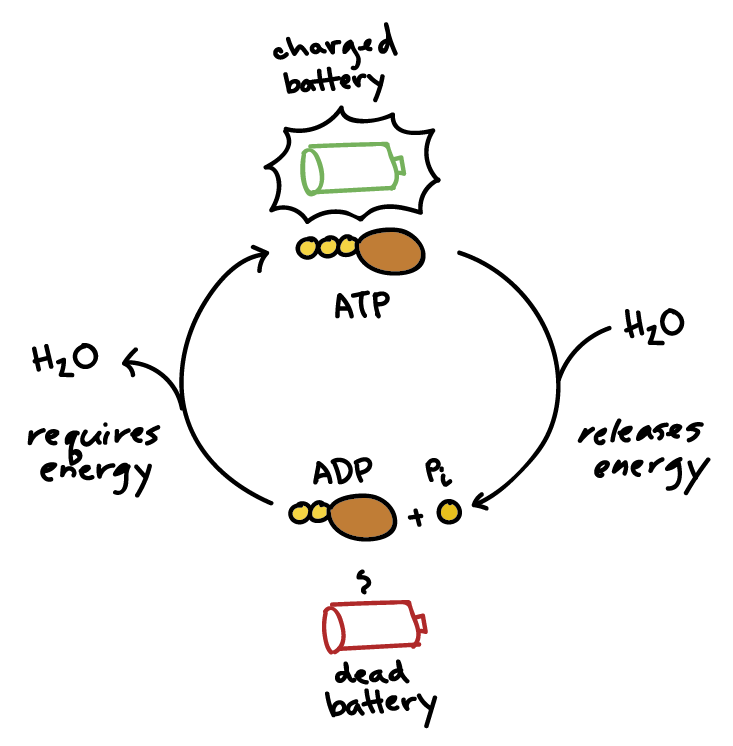
ATP stores chemical energy
in the covalent bonds between phosphate groups
phosphates are negatively charged and hence
require high amounts of energy to keep in place
when ATP is hydrolysed
the terminal phosphate is released an coenzyme is converted to its unloaded form
chemical energy released by ATP hydrolysis is used by
an enzyme to catalyse a metabolic reaction within the cell
energy transfer
cell respiration is the controlled
release of energy from the breakdown of organic compounds to produce ATP
the main organic compound used for cell respiration is
carbohydrates
lipids and proteins can also be digested
in the process of cell respiraiton
lipids produce more energy per gram however
they are harder to digest and transport
proteins can produce the same amount of energy as carbs but
also produce toxic nitrogenous waste
energy sources
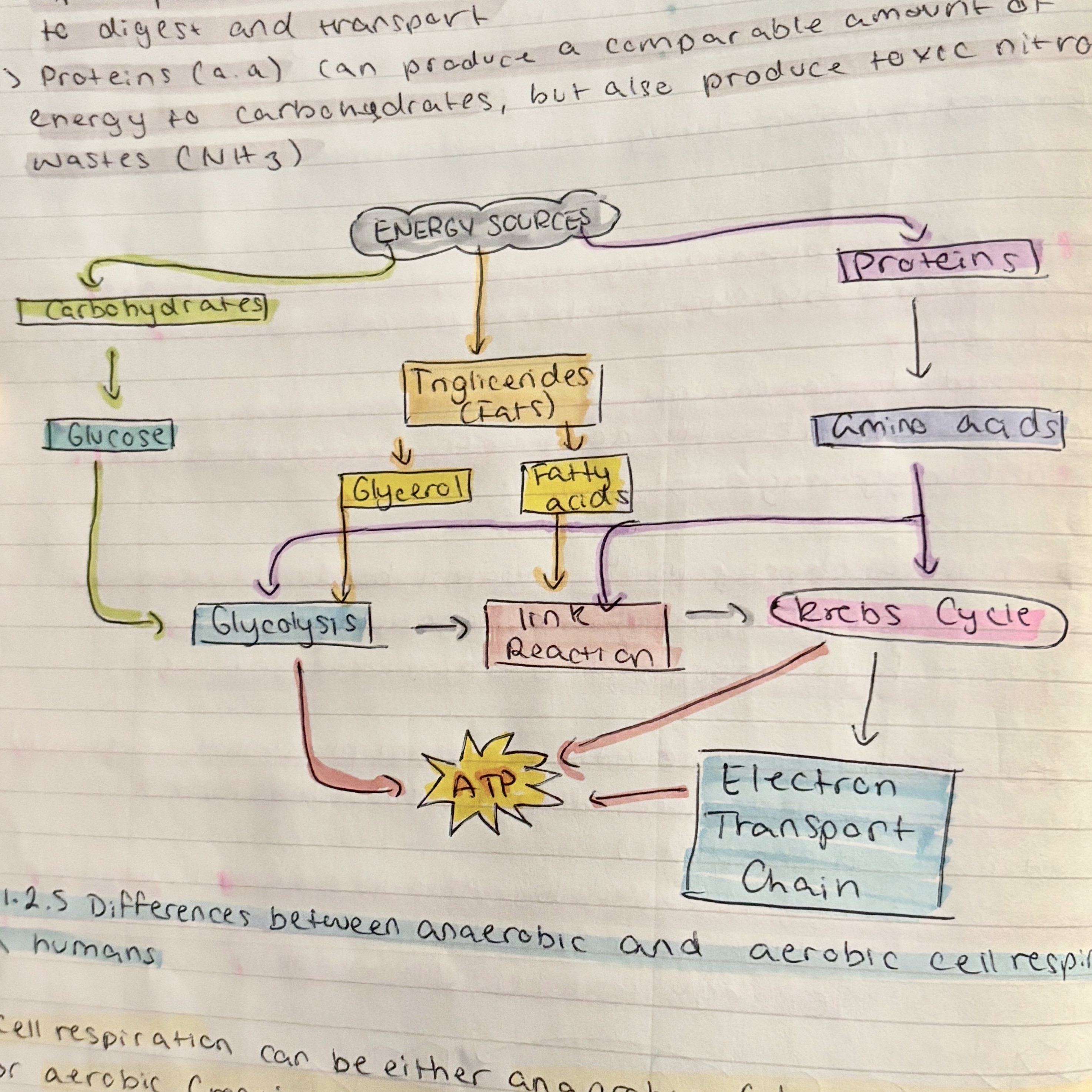
cell respiration can either be
anaerobic or aerobic
the two forms of cell respiration anaerobic and aerobic
differ in the products that are formed, where the reaction occur ad the overall ATP yield
not all respiratory substrates can undertake
both form of respirations
fatty acids can only be digested
aerobically
anaerobic respiration begins with
the process of glycolysis
glycolysis
glucose is partially broken down into two pyruvate molecules with a small yield of ATP
certain amino acids and glycerol may feed into
the glycolytic pathway and produce pyruvate anaerobically
the absence of oxygen the pyruvate molecules are
fermented to form lactic acid in animals or ethanol and carbon dioxide in plants
the anaerobic processes of glycolysis and fermentation both occur within
the cytosol of the cell
aerobic respiration also begins with the process of glycolysis but
oxygen is then used to completly break down the pyruvate for a much larger ATP yield
in aerobic respiration the pyruvate is transported to the mitochondria and
is broken down into carbon dioxide and water
the complete breakdown of pyruvate involves
the link reaction, the Krebs cycle and the electron transport chain
both anaerobic and aerobic respiration use
digestion and oxidation of organic molecules to synthesise ATP
glycolysis is common to both
anaerobic and aerobic respiration
while sugars are the main respiratory substrate
lipids and proteins can both be converted into usable intermediates