atomic models
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms
john dalton
dalton atomic theory
elements made of atoms
atoms of one element are identical while atoms of different elements are different
atoms can’t be subdivided, created, or destroyed
in chemical reactions, atoms are combined, separated, and rearranged
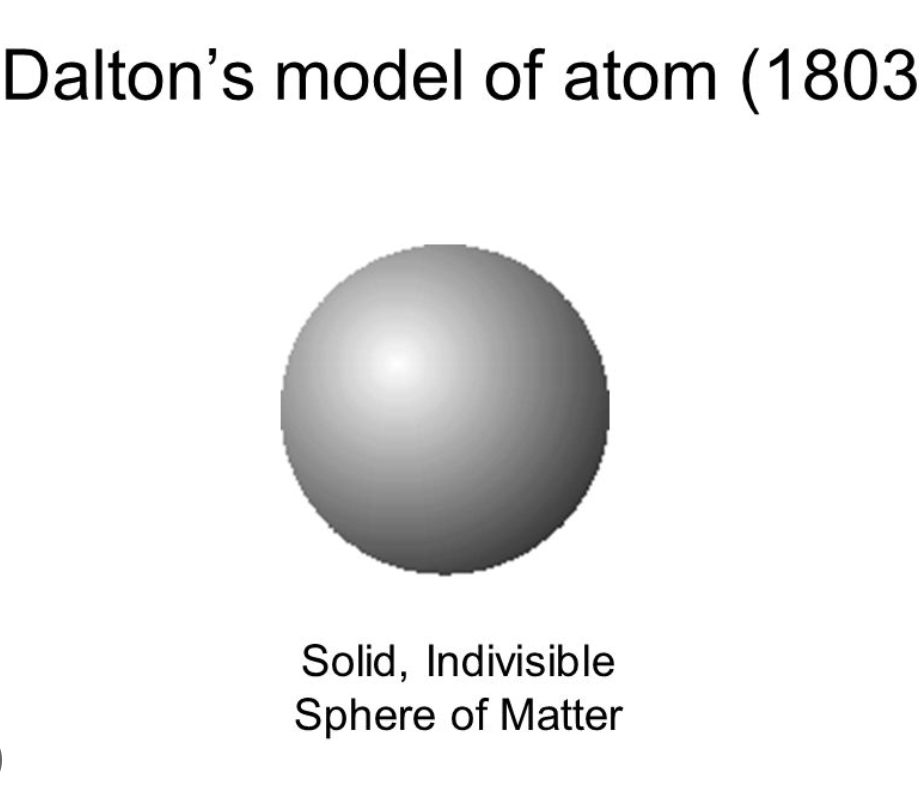
solid sphere model
atoms are tiny, indivisible solid spheres with no internal structure
J.J. thomson
discovered electrons using cathode ray tubes
atoms are divisible and contain smaller particles within them
plum pudding model
sea of positive charge (pudding)
dispersed electrons (plums)

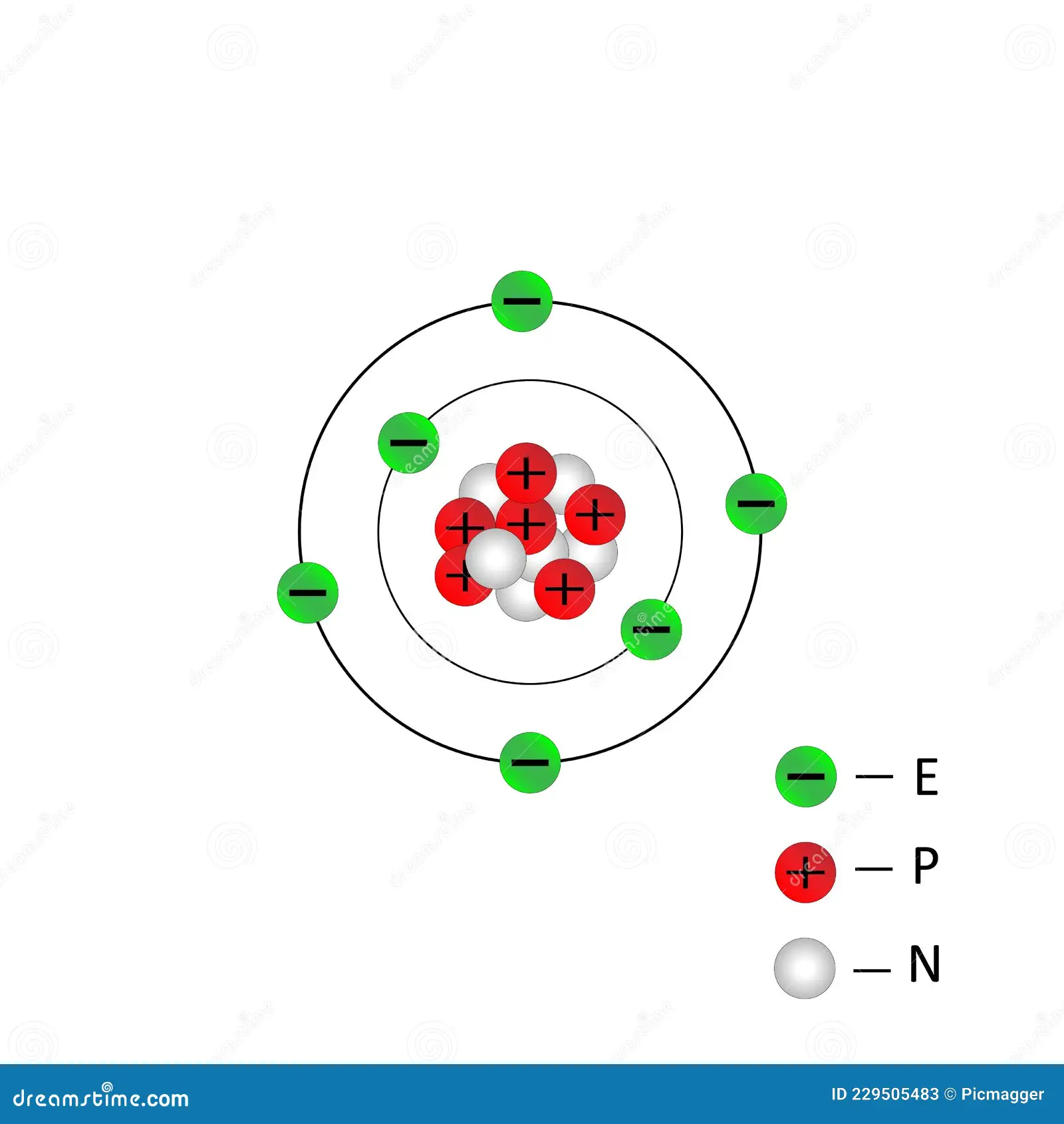
ernest rutherford
gold foil experiment
nuclear model
nucleus in center
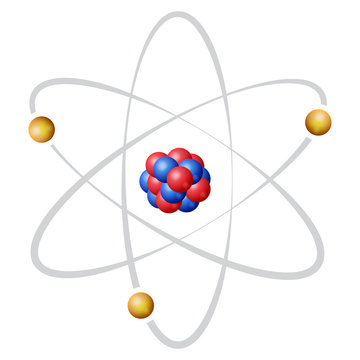
niels bohr
planetary model
nucleus in center
electrons orbit around nucleus
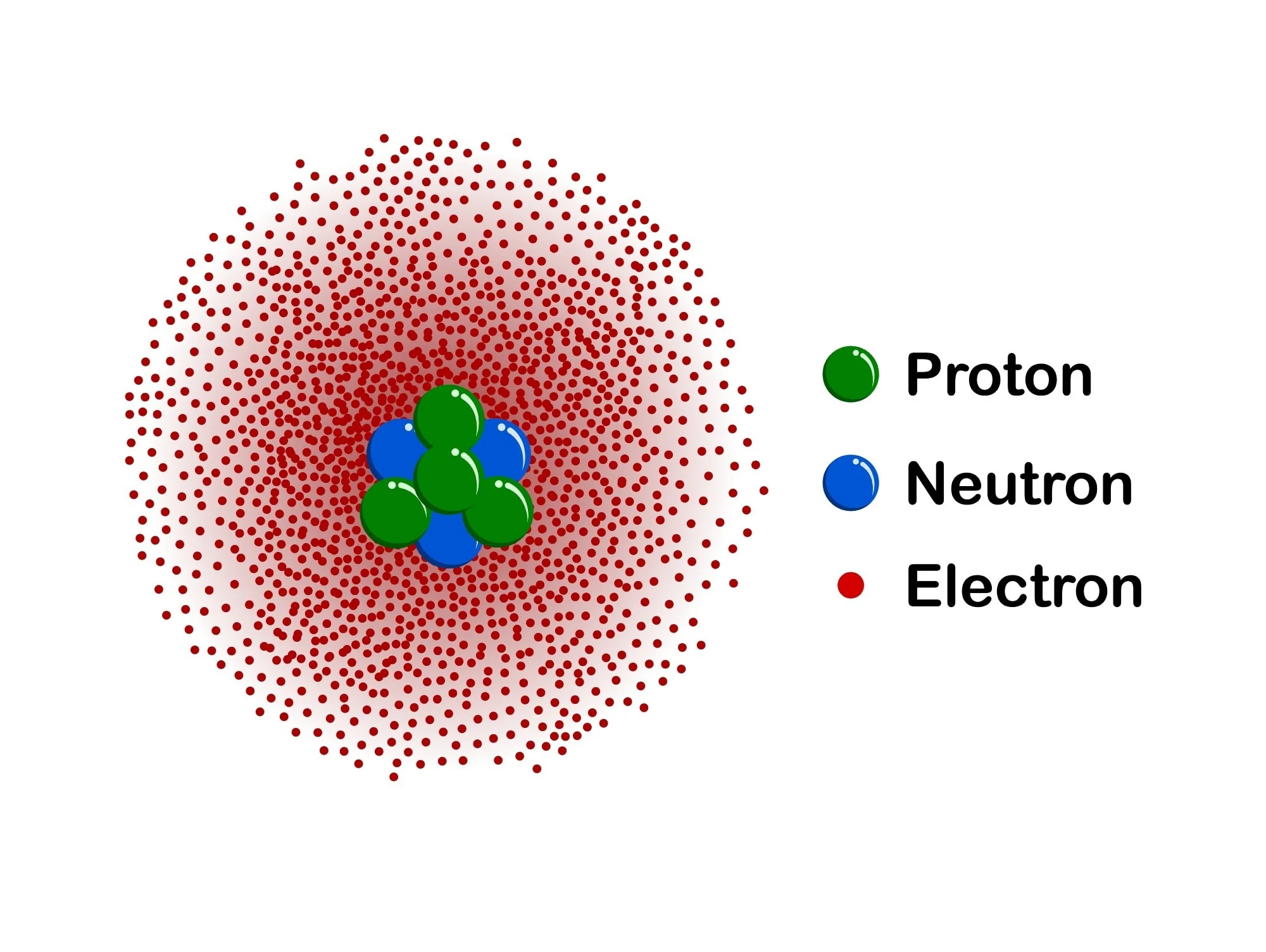
erwin schrödinger
quantum model
nucleus in center
cloud of electrons