structure of an atom
1/7
Earn XP
Description and Tags
ccc 2nd year science
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
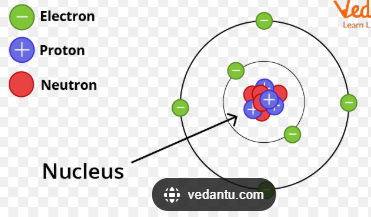
an atom is
the basic building blocks off all things
describe the properties of protons
location in the atom - nucleus
relative mass - 1
relative charge - +1
describe the properties of neutrons
location in the atom - nucleus
relative mass - 1
relative charge - 0
describe the properties of electrons
location in the atom - shells
relative mass - basically none
relative charge - -1
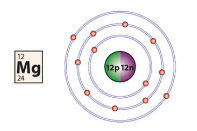
a diagram of an atom
what info does the atomic number of an atom give us
the amount of protons and electrons e.g. aluminums’s atomic number is 13 so there is 13 protons and 13 electrons
what info doe the mass number of an atom give us
when you minus the atomic number from it it gives you the number of neutrons e.g. aluminums mass number is 27 so 27 - 13 = 14 so the number of neutrons is 14
how do you draw a diagram of an atom
find out the atomic and mass number
draw a circle and write the amount of neutrons and protons in this circle
the amount of electrons in the atom varies the amount of circles around the centre circle
e.g. there are 16 electrons
there can be 2 electrons in the first shell, 8 in the second and 8 in the third