Organic Chem - Alcs and Ethers and Polymers
1/13
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
alcohol
a compound with an –OH (hydroxyl group) attached to a carbon group
ether
an - O - as its functional group
Alcohols can vary and be classified depending on where their
–OH group is located
Primary alcohol
hydroxyl group is bonded to terminal (first) carbon
secondary alc
hydroxyl group is bonded to a carbon with two alkyl groups
Tertiary alcohol
hydroxyl group is bonded to a carbon with three alkyl groups
Naming Alcohols
Identify the longest carbon chain
If there is only one –OH group, the compound has the suffix –ol. If more, use –diol, or -triol
Number the parent chain from the end so that the –OH group is attached to the carbon atom with the lowest possible number
Identify any other branches and their locations.
Properties of Alcohols
higher boiling points than their corresponding alkane because of the –OH group
-OH group makes alcohol more polar
The longer the alkyl portion (C-H), the less polar the molecule
Naming Ethers
Add –oxy to the prefix of the smaller hydrocarbon group and join it to the alkane name of the larger carbon group
A number is used to indicate which carbon of the larger chain the oxygen has attached to. • Note: number the larger chain from the O outward.
Properties of Ethers:
Structure is similar to that of water and alcohol
It is more polar than the corresponding alkane group, but less polar than alcohol.
Higher boiling point than their corresponding hydrocarbons, but lower boiling point than similar alcohol
Form good solvents for organic substances as they readily mix with both polar and non-polar substances
order of priority (12)
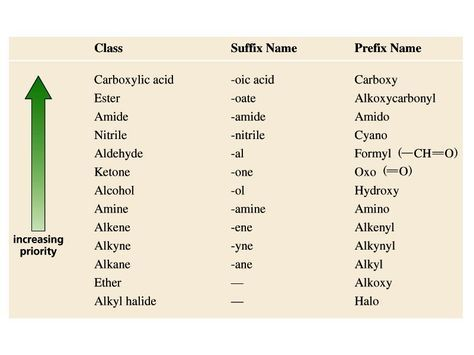
hydroxyl
OH group
what do you call it when only one OH is on a benzene
phenol
what do you call it when more than one OH is on a benzene
use benzene for the main name