adaptive immune system
1/27
Earn XP
Description and Tags
week 1 block 2 ctb
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
28 Terms
adaptive immune system components
cellular components
B lymphocytes (including plasma cells)
cytotoxic T lymphocytes (Tc)
helper T lymphocytes (TH)
regulatory T lymphocytes (Treg)
APCs
memory cells: B/T lymphocytes
humoral component:
ABs (immunoglobulins, Ig- various classes)
T cells and B cells
B cells: secrete ABs (immunoglobulins, Ig)
TC: specifically kill infected cells and some tumour cells
TH: secrete cytokines
2 arms of adaptive immunity
both B and T cells have receptors for specific antigens on the cell surface
only one version of a receptor on each individual cell
millions of different receptors in the population of cells
antigen receptor on B cells is membrane-bound immunoglobulin
importance of the fact that there is onlt one specificity of receptor on any one cell
can control which receptors are present by controlling cells
can eliminate receptors of particular specificity by eliminating cells
can produce more of the receptor by stimulating multiplication of the appropriate cells
B cell activation: plasma cells
co-operation with TH cells is required to activate B cells
when correct antigen binds to antigen-receptor (surface Ig) on B cell surface, cell is activated and starts producing and secreting the same AB at a high rate
each activated B cell and its progeny produces AB with one sort of antigen binding site
importance of cell signalling
first step: binding of antigen to the surface Ig
must change the behavious of the cell: activating it by changing gene expression
signals must pass from plasma membrane to the nucleus: by phosphorylation of target proteins by kinases, changes in Ca, etc
intra-cellular signalling pathways are important in controlling immune response
adaptive humoral immunity
humoral immunity: aspects of immunity-mediated by molecules in extracellular fluid as opposed to cell-mediated immunity
term often used to refer to AB-mediated immunit
AB binding to an antigen can:
block a pathogen from causing harm
opsonisation: mark a pathogen for phagocytosis
activate complement system
multiple AB binding
many different ABs can bind to separate parts of the same antigen known as epitopes
some of these ABs will have higher affinity for antigen than others
all usual inter-protein binding forces apply (charge interactions, hydrophobic interactions, etc) as well as shape complementarity
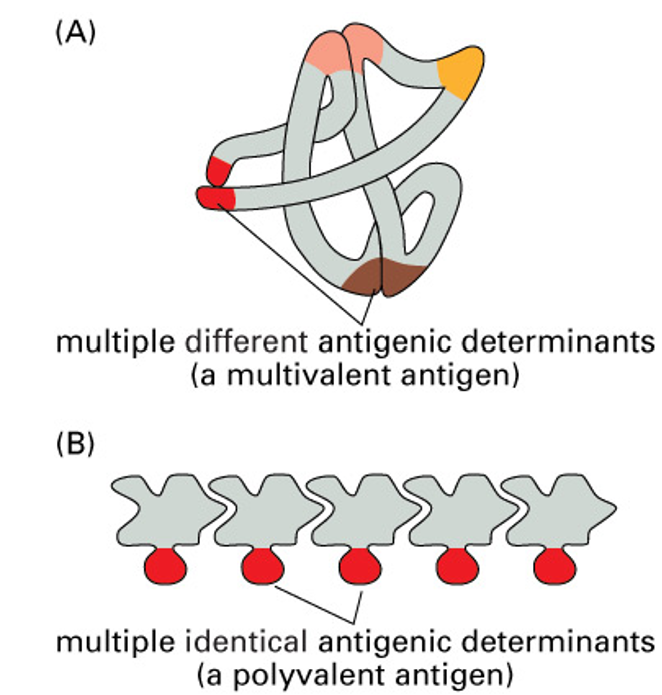
epitopes
large molecules can have multiple structural features (epitopes) that act as antigenic determinants
usually the molecule has different structural features
for some molecules (eg polysaccharides), the same structure is present in multiple copies
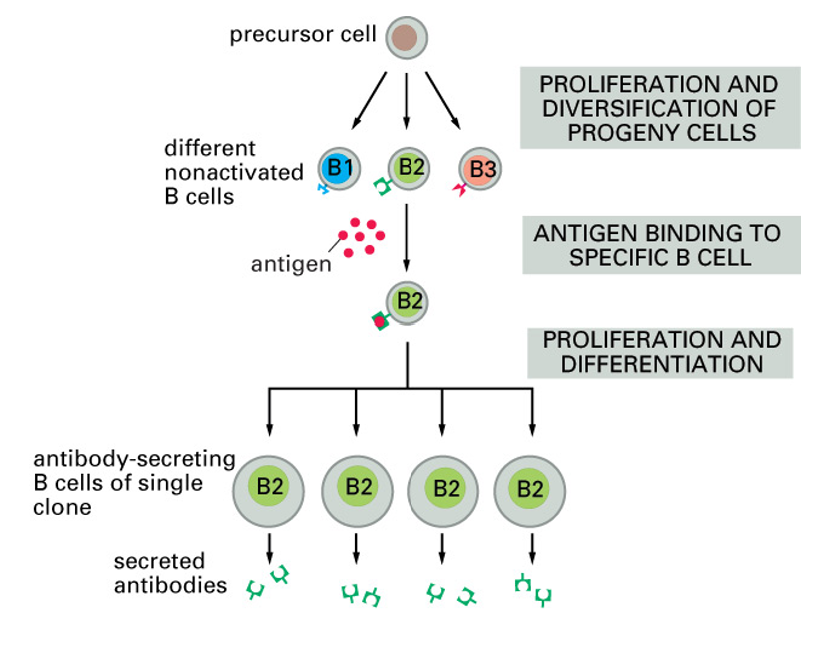
clonal selection
B cells producing the right AB are selected from a big range of B cells
each stimulated B cell produces a clone of cells producing AB that binds the same antigen
a particular antigen may activate hundreds of different clones
immunological memory
selective stimulation of B cells recognising antigen means that the AB produces reacts specifically with that antigen
can also be applied to T cells
pathogen-reactive lymphocytes are therefore selectively expanded, resulting in increased immunity (can be long lasting)
activated TH are also needed for B cell activation
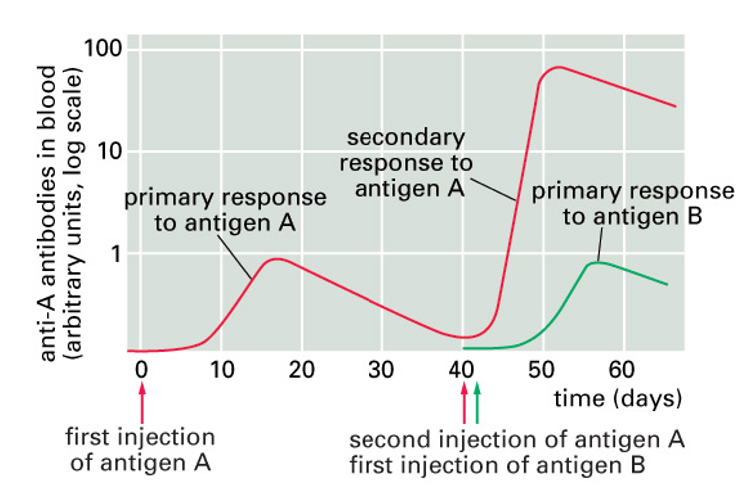
primary and secondary AB responses
after antigen exposure, only small fraction of B cells will specifically recognise it
will be triggered to secrete their Ig (antibody)
these cells also proliferate: some of the cells formed will persist and form memory cells
next time more cells recognise the antigen
log scale: almost 100x as much AB can be produced in the secondayr response and more quickly
specificity: different antigen injected later only produces the much smaller primary response
antibody classes
IgG
IgE
IgM
IgA
IgD
AB class switching
involves rare event: loss of DNA within genome very rarely occurs in any mammalian cells
lymphocytes use changes in their genomic DNA for generating different antigen-binding sites as well as class switching
T cell function
T cells all possess receptors which have large range of specificites (like surface Igs - B cell antigen receptors)
due to highly diverse AA sequences of the variable (antigen-binding) region
2 main types of T cell:
TC : most of which have the cell surface protein CD8
TH : most of which have the cell surface protein CD4
TH cells
TH activate other cells, including B cells and macrophages, by cell-cell contact and by secreting cytokines
TH1 cells secrete cytokines (interferon γ) that mainly activate virally infected cells, macrophages and other T cells
TH2 cells secrete (interleukins) that mainly activate B cells
some CD4+ (Tregs) have inhibitory roles
cytotoxic T cells
cytotoxic T cells are central to the cell-mediated arm of adaptive immunity
almost all cell types can present antigen on MHCI molecules on their cell surface if infected
when cytotoxic T cell recognises foreign antigen presented on MHCI molecules, it proliferates
TH cells influence this process
activated TC cells produce the pore forming proteins perforn and granulysin, as well as granzymes (proteases)
causes apoptosis of the target cell
TCR (T cell receptor)
TCRs are αβ heterodimers with each monomer about 280 AAs in size
each of the monomers has 2 immunoglobulin-like domains, one variable and one constant
variable domains contain complementarity-determining regions
TCRs are associated with CD3 complex, which is involved in cell signalling
antigen presentation
T cells won’t recognise antigens in solution: only peptides ingested, processed and presented on MHC complexes by other cells
MHC proteins
MHC proteins were named from their crucial role in controlling transplantation rejection
MHC proteins present antigens (foreign peptide) on cell surface
T cell receptor on the surface of T cells interacts with both the antigen and the MHC protein presenting it
MHC incompatibility leading to transplant rejection of unmatched tissue accounts for search for ‘compatible’ donors
other interactions between APCs and T-cells do occur, but only the TCR/antigen/MHC interaction gives the high specificity
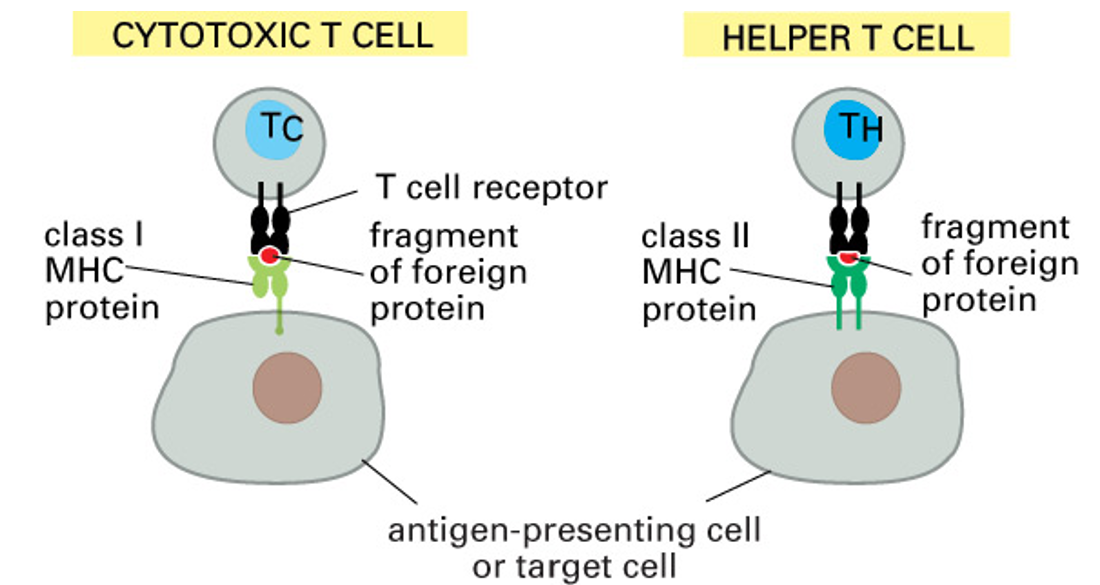
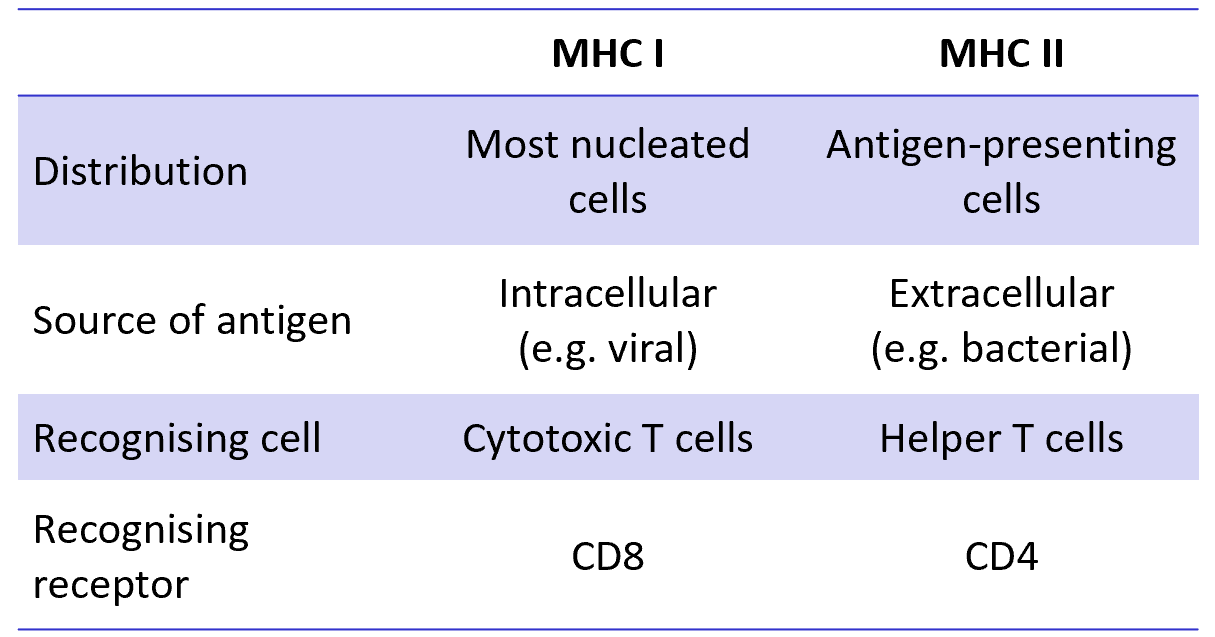
class I MHC proteins
present on most cells
recognised by CD8+ cytotoxic T cells
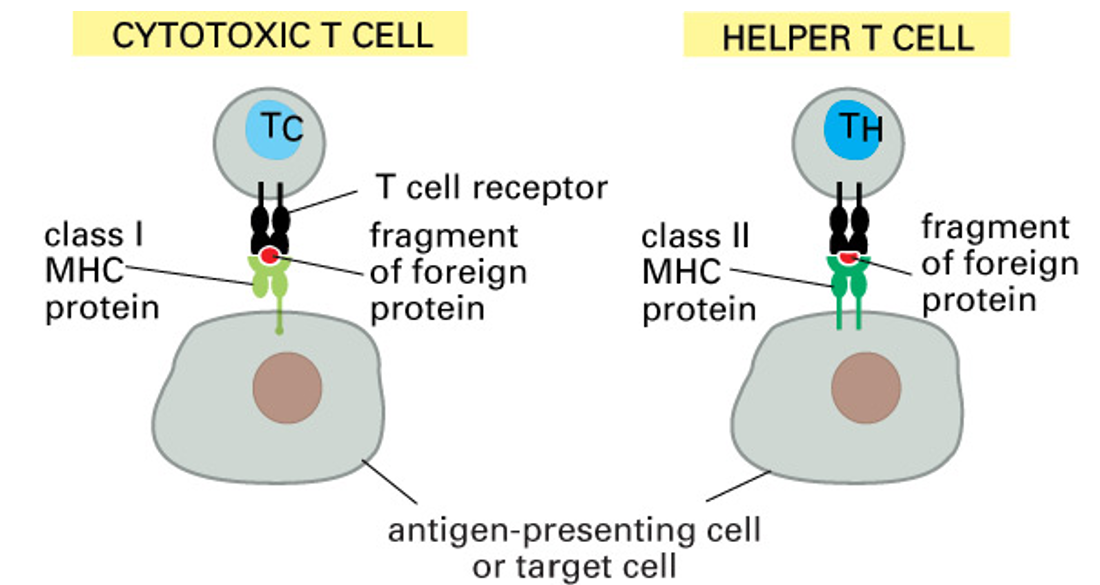
class II MHC proteins
present on APCs
recognised by CD4+ helper T cells
class I MHC protein structure
MHCs are 4 domain αβ heterodimers
in class I MHC, the β2 microglobulin is an invariant chain non-covalently attached to the α chain
binding site for the antigenic peptide is formed from 2 domains of the α chain
peptides are derived from the cell cytoplasm
peptides derived from cell cytoplasm may include those from proteins of intracellular viruses
class II MHC protein structure
class II MHC protein is αβ heterodimers
2 similarly sized polypeptide chains
overall domain structure has clear similarities to class I MHC
the binding site for peptide antigens is formed from both chains combined
peptides are derived from ingested material
CD4and CD8 accessory molecules
CD4 and CD8 bind to non-variable parts of MHC proteins and promote interaction between T cells and target/presenting cells
class I MHC presents antigens from within any cell (viral peptides from an infected cell) and is recognised by CD8+ cytotoxic T cells which will kill the infected cells
class II MHC presents antigen ingested and processed by a macriohage, dendritic cells or B cells and stimulates CD4+ TH cells
class I and class II MHC protein tables
MHC protein variability
several hundred different allelic variants of class I and class II MHC molecules have been identified in humans
encoded at MHC genetic locus, crucial to immune response
capable of presenting an enormous array of peptides to T cells (their broad specificity is very different to high specificity of antigen binding to Ig/TCR)
MHC molecules present in individuals do influence susceptibility to disease, presumably through different efficiencies in presenting peptides from pathogens)