Unit 4 Organic Chemistry
1/86
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
Functional group
a specific group of atoms or bonds within a compound that is responsible for the characteristic chemical reactions of that compound
Alkane
Single carbon bond - saturated
Alkene
Double carbon bond - unsaturated
Alkyne
Triple carbon bond - unsaturated
Alchohol
Carbon bonded to an OH
Haloalkane
Halogen bonded to carbon chain
Amine
NH2 bonded to carbon chain
suffix: amine
occasional prefix: amino
Aldehyde
Carbonyl
attatched to a hydrogen at the end of the carbon chain
suffix: al
prefix: oxo
Ketone
Carbonyl
that’s bonded with another carbon
always in the middle of the carbon chain, as two carbons are bonded
suffix: one
prefix: oxo
Carboxylic acid
Carbonyl
the carbon is bonded to an hydroxyl group (OH)
suffix: oic acid
Ester
Carbonyl
bonded with an oxygen then bonded with normal R group.
basically replaces the Hydrogen in carboxylic acid, and it connects two chains
suffix: “oate” goes to the side chain next to the carbonyl, “yl” next to the oxygen side chain
prefix: -R-oxycarbonyl
Amide
Carbonyl
then single bonded with a Nitrogen (NH2)
suffix:amide
Nitrile
Carbon triple bonded with Nitrogen (N)
suffix: nitrile
prefix: cyano-
Haloalkane primary functional group definition
Out of the 4 carbon bonds possilbe, one is the halogen, two is hydrogen, and the last is an alkyl chain (carbon)
Basically, one alkyl chain (1 carbon most times)

Haloalkane secondary functional group definition
Out of the 4 carbon bonds possible, one is the halogen, only one is the hydrogen now, now there are two alkyl chains attached
Basically, two alkyl chain (2 carbons most times)
Haloalkane secondary functional group definition
Out of the 4 carbon bonds, one is the halogen, no hydrogens now, three alkyl chains attached
Basically, three alkyl chain (3 carbons most times)
Naming alchohol rule
In numbering the carbon chain, the hydroxyl group takes priority over any alkyl side chains.
Order the functional groups from highest priority to lowest priority when naming
Alpha carbon is always at the carboxylic acid end
Omega carbon is at the other end (CH3 end)
Alpha carbon is always at the carboxylic acid end
Omega carbon is at the other end (CH3 end)
omega 3s have carbon double bond at the 3rd last carbon from the omega carbon
omega 6s have carbon double bond at the 6th last carbon from omega carbon
Tips for Reaction Pathways
Oxidation always adds oxygen or removes hydrogen (alcohol → aldehyde → acid).
Reduction is the reverse.
Addition reactions add atoms across a double bond (alkene), breaking a double bond
Substitution swaps groups (haloalkanes).
Elimination removes small molecules (like H₂O or HCl), creating a double bond
Reaction Pathway, Alkane
→ Haloalkane
Reaction: Free radical substitution
Conditions: Halogen + UV light
Reaction Pathways, Alkene
→ Alkane
Reaction: Hydrogenation (addition)
Conditions: H₂, Ni catalyst, heat→ Haloalkane
Reaction: Electrophilic addition
Conditions: HX (e.g. HBr)→ Dihaloalkane
Reaction: Addition
Conditions: X₂ (e.g. Br₂)→ Alcohol
Reaction: Hydration (addition)
Conditions: Steam, H₃PO₄ catalyst, heat
Reaction pathways, Haloalkane
→ Alcohol
Reaction: Nucleophilic substitution
Conditions: Dilute NaOH, heat→ Nitrile
Reaction: Nucleophilic substitution
Conditions: KCN in ethanol, heat under reflux→ Amine
Reaction: Nucleophilic substitution
Conditions: NH₃ in ethanol, heat under pressure→ Alkene
Reaction: Elimination
Conditions: Ethanolic KOH, concentrated NaOH, heat
Reaction Pathways, Primary Alchohol
Aldehyde (from primary alcohol)
Reaction: Oxidation
Conditions: Acidified K₂Cr₂O (strong oxidisting reagent)₇, heat,
Reaction Pathways, Secondary Alcohol
→ Ketone (from secondary alcohol)
Reaction: Oxidation
Conditions: Acidified K₂Cr₂O₇ (strong oxidising agent)
Reaction Pathways, Aldehyde
→ Carboxylic acid
Reaction: Oxidation
Conditions: Acidified K₂Cr₂O₇, reflux→ Alcohol
Reaction: Reduction
Conditions: NaBH₄
Reaction Pathways, Carboxylic acid
→ Ester
Reaction: condensation
Conditions: Alcohol + conc. H₂SO₄, reflux
→ Amide
With: Amine (R–NH₂)
Reaction: Condensation (eliminates H₂O)
Conditions: Heat (often high), or use acyl chloride intermediate
Reaction Pathways, Nitrile
→ Amine
Reaction: Reduction
Conditions: H₂ with Ni catalyst
Reaction Pathways, Amine
→ Amide
Reaction: Condensation
Conditions: Reaction with carboxylic acid derivative (acid chloride or ester)
Volatility of an organic compound
Organic compounds that contain
weak intermolecular forces
evaporate more easily and are
therefore more volatile than
organic compounds that contain
strong intermolecular forces.
Important rule when it comes to solubility
Like dissolves like, so the molecule has to be polar to be dissolved water, since water is polar
What are two factors that affect solubility
The type of functional group present in the compound and the length of the carbon chain.
longer carbon chain - loses solubility
Solubility of Organic Compounds (in Water):
Solubility depends on polarity and ability to form hydrogen bonds.
Alcohols: Very soluble (small ones) — can donate & accept H-bonds via –OH.
Aldehydes & Ketones: Moderately soluble — accept H-bonds via C=O only.
Carboxylic Acids: Highly soluble (small ones) — strong H-bonding (donor & acceptor).
Hydrocarbons (alkanes, alkenes, alkynes): Insoluble — nonpolar, no H-bonding.
Longer hydrocarbon chain → lower solubility (nonpolar portion dominates)
Some common acid reactions
Acid + base —>
Acid + metal —>
Acid + carbonate —>
Acid + base —> Salt + H20
Acid + metal —> Salt + Hydrogen
Acid + carbonate —> Salt + H20 + C02
What is maltose
A disaccharide that consists of two glucose molecules
Lactose
A disaccharide that consists of one glucose and one galactose molecule together
Sucrose
A disaccharide that consists of one glucose and one fructose molecule together
Difference between alpha and beta glucose
Alpha glucose has the OH at carbon 1 pointing downwards
Beta has the OH pointing upwards
What is amylose
Amylose is a polysaccharide (a carbohydrate polymer).
It is made of α-glucose monomers linked mainly by α-1,4-glycosidic bonds.
Fatty acid
A carboxylic acid group at one end (–COOH),
A long hydrocarbon chain (usually 12–22 carbons).
Saturated fatty acid
Monounsaturated fatty acid
Polyunsaturated fatty acid
Saturated fatty acid → no double bonds (all single C–C).
Monounsaturated fatty acid → one double bond (C=C).
Polyunsaturated fatty acid → two or more double bonds (C=C).
Triglyceride
Triglyceride = complete molecule made of glycerol + 3 fatty acids.
Primary Structure of Protein
Sequence of amino acids in a polypeptide chain
Held by peptide bonds
Determines all higher levels of structure
Secondary structure of protein
Local folding due to hydrogen bonding along backbone
Two main shapes:
α-helix (coiled)
β-pleated sheet (zigzag, sheet-like)
Tertiary Structure
Overall 3D folding of a single chain
Stabilised by:
Disulfide bridges (covalent S–S)
Hydrogen bonds
Ionic bonds
Hydrophobic interactions
Gives the protein its unique shape & function
Quaternary Structure
Two or more polypeptide chains joined
Can be identical or different subunits
Examples: haemoglobin, insulin, DNA polymerase
Important for proteins with multiple functional units
Difference between secondary and tertiary structures
Secondary = local shapes (helix, sheet) formed by backbone H-bonds.
Tertiary = overall 3D folding of the whole chain, arranging helices/sheets together via R-group interactions.
Explain why the effectiveness of an enzyme is usually
over only a narrow pH and temperature range.
The shape of an enzyme is critical in determining its function, and as enzymes are proteins, their three-dimensional shape is affected by pH and temperature. A change in pH and/or temperature will disrupt the attractions in the protein (enzyme) that determine its tertiary shape
How to tell if a fatty acid is unsaturated or saturated
count the total number of carbons, multiply by 2.
Because of double oxygen (-2 bonds)
So if the number of hydrogens matched 2 * carbons, then it is saturated (single bonds)
If it is not equal, then every double bond reduced the hydrogen count by 2 (unsaturated)
Explain the difference between omega-3 fatty acid and omega-6 fatty acid
The term ‘omega’ refers to the last carbon in the fatty acid chain. The number 3 or 6 indicates the position of the double bond in the unsaturated fatty acid in relation to this carbon. That is, the double bond in omega-3 fatty acids is the third carbon along from the omega carbon.
What are things that optimise yield
reaction conditions
purity of product
multi step reaction pathway
What is retrosynthetic pathway
a method in organic chemistry where chemists plan the synthesis of a complex target molecule by working backward from the desired product to simpler, more readily available starting materials.
Biodiesel
A mixture of long carbon chain esters made from natural sources
Transesterification
alcohol + ester —> different alcohol + different ester
A type of reaction that changes a particular ester into a different ester
In a fuel cell/galvanic cells, what type of electrodes are used if we are using gaseous reactants
inert electrodes so that it doesn’t interact
Alkaline hydrogen fuel cell produces
water liquid as a product
Acidic hydrogen fuel cell produces
water vapour as a product
Green chemistry
Green chemistry describes principles producers use to manage the environmental impact of a chemical process. This helps them focus on methods that reduce and eliminate the hazardous impacts of chemical synthesis
Atom economy
Atom economy is a measure of the amount of reactants that end up not just as products, but as the desired product.
what are the three elements of a synthesis process that producers should consider according to green chemistry
Raw materials, waste products, method of production
Examples for raw materials
Producers should consider using recyclable materials to reduce the amount of reactants needed.
Producers should consider using non toxic materials to reduce harm to consumers and the environment.
Producers should consider using biodegradable materials to reduce environmental harm.
Producers should consider avoiding the use of volatile organic compounds as they lead to the formation of ozone and smog and a decrease in air quality.
Examples for Method of Production
Producers should consider the energy sources used for production, such as replacing non-renewable energy sources with renewable ones to reduce environmental impact.
Examples of waste products
Producers should consider whether they are creating toxic or harmful waste materials to guide their disposal process.
Producers should consider ensuring the waste is either biodegradable or recyclable in order to minimise environmental harm.
Producers should consider reducing the total volume of waste material in order to reduce environmental impact.
Molecular Manufacturing
The process of carefully positioning reactants in a reaction to make products that have a specific structure, shape or composition
Three methods for molecular manufacturing
Orientation effect
Manipulating structures (changing bonds or atoms)
Protecting other reactive functional groups in a molecule
Bottom up and top down approach in molecular manufacturing
The bottom-up approach to molecular manufacturing involves the creation of nanoparticles by putting together smaller particles whereas the top-down approach involves the creation of nanoparticles by breaking down larger molecules.
Benefits/weakness of bottom up and top down
The bottom-up approach is highly efficient as we can select the exact bonds and structures that form whereas the top-down approach is less efficient as it uses a lot of energy and we can't choose the exact product that forms, resulting in large amounts of waste material.
Why do we produce alcohols using fermentation instead of alkene hydration
Ethanol is produced by fermentation of glucose rather than from an alkene because fermentation uses renewable, low-energy, carbon-neutral biological feedstocks, whereas ethene comes from non-renewable fossil fuels and requires energy-intensive conditions.
12 principles of Green Chemistry
Prevent waste
Maximise atom economy.
Design less hazardous chemical syntheses/waste products
Design safer chemicals and products
Use safer solvents and reaction conditions.
Increase energy efficiency
Use renewable raw materials
Avoid chemical derivatives.
Use catalysts, not stoichiometric reagents.
Design chemicals and products to degrade after use
Analyse in real time to prevent pollution
Minimise the potential for accidents
Physical vs Chemical properties
“If I measure this, does the substance change into something new or make other products?”
If no → physical property
If yes → chemical property
Physical Property
A characteristic that can be observed or measured without changing the substance’s chemical identity.
Chemical Property
A characteristic that describes a substance’s ability to undergo a chemical change or reaction, forming new substances.
Primary and Secondary Cell
Electrochemical cells (batteries)
Primary - Used once and then discarded (irreversible reactions)
Secondary - Can be recharged many times (reversible reactions) - lithium ion battery
Difference to fuel cell is that they don’t need a external source supplying the reactants to the cell
Mass Spectrometry for aldehydes
For small aldehydes (≤ propanal) → base peak ≈ 29
For butanal and longer → base peak ≈ 44
Mass spectrometry for ketones
base peak should be 43 m/z
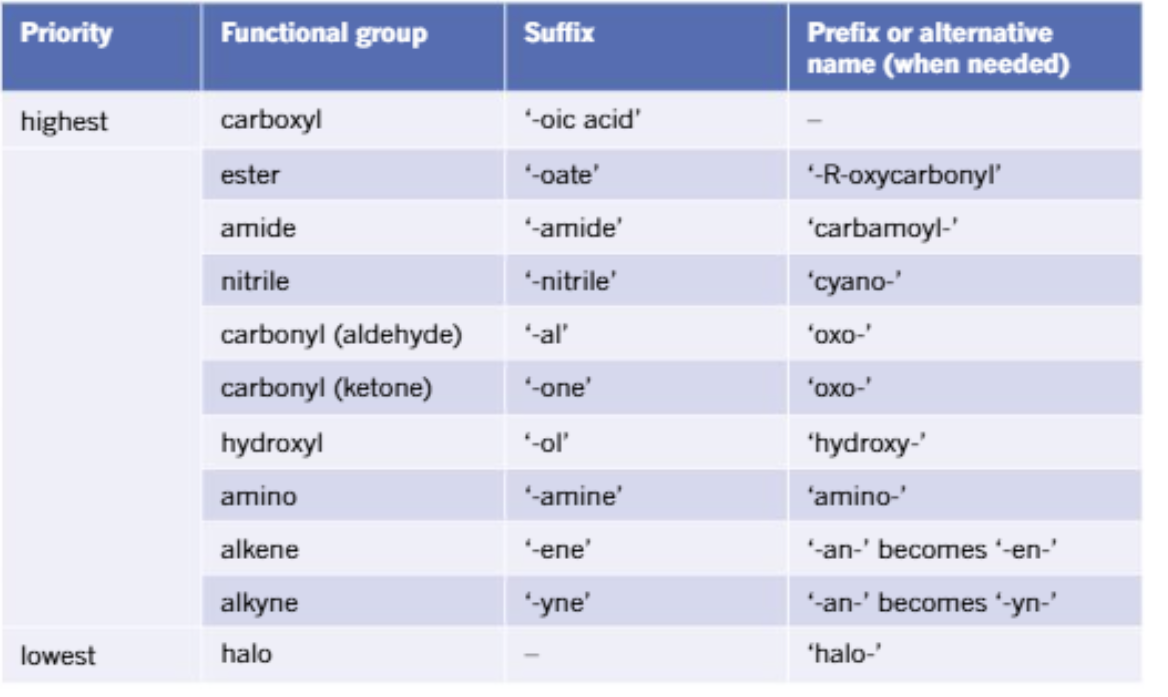
Fermentation reaction of alcohol
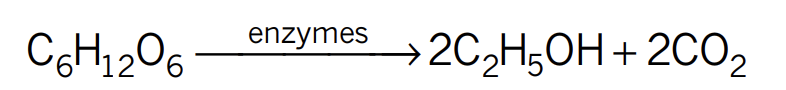
Combustion of ethanol reaction
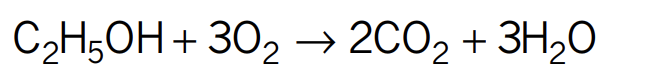
Photosynthesis chemical reaction
biochemical fuel
A biochemical fuel is a fuel derived from plant materials such as vegetable waste, sugar cane or vegetable oil.
how to calculate amount of CO2 per coloumb
dividing the number of moles of carbon
dioxide by the number of electrons produced.
Disadvantages of Green Chemistry
Biodiesel made from triglycerides (oil extracted from grains) - Using land to grow grain for biodiesel means there is less land available to grow food
Biofuel made from glucose - The companies that produce the biofuel are likely to buy the most fertile land thereby forcing farmers to use land with poorer crop yields.
Much of the land used to grow biofuels requires trees to be cut down.
What is Mass Spectrometry
Mass spectrometry differentiates molecules based on total molar mass and the mass of different molecular fragments. Structural isomers have the same molar mass, and many of the fragments that would be produced by the different isomers of C4H9Cl are identical, making it difficult to distinguish between isomers of this molecule using mass spectrometry. Infrared spectrometry would be a better alternative. (
In an electrolysis where it just shows you line to a voltage, how can you identify which is the cathode and anode
The electrode connected to the positive terminal (longer line) of the 6.0 V source is the anode.
The electrode connected to the negative terminal (shorter line) is the cathode.
What is the role of the electrolyte in hydrogen fuel cells
The electrolyte lets ions move internally to balance the flow of electrons through the external circuit, preventing charge build-up and allowing continuous redox reactions.
PEM (acidic) fuel cell:
Electrolyte allows H⁺ ions to move from the anode → cathode.
Reaction summary:
Anode:
H₂ → 2H⁺ + 2e⁻Cathode:
O₂ + 4H⁺ + 4e⁻ → 2H₂O
Alkaline fuel cell:
Electrolyte allows OH⁻ ions to move from the cathode → anode.
Reaction summary:
Anode:
H₂ + 2OH⁻ → 2H₂O + 2e⁻Cathode:
O₂ + 2H₂O + 4e⁻ → 4OH⁻
In the acidic fuel cell, water is just a by-product.
In the alkaline fuel cell, water is part of the ion transport cycle — it’s used and regenerated.
why is oxidisation loss of hydrogen/gain of oxygen
When we say “carbon loses electrons,” we mean:
➡ The shared electrons in a bond are being pulled closer to oxygen (since oxygen is more electronegative).
So carbon loses control or ownership of those electrons.
That’s what counts as “losing electrons.”
and thats why gain of oxygen is oxidisation and loss of hydrogen is oxidisation
Biodiesel production from FFA-rich oils (the usual “two-step” process)
We start with waste oils or fats that contain a lot of free fatty acids (FFAs) and triglycerides.
FFAs can’t directly undergo transesterification efficiently — they form soap instead.
So, we first convert those FFAs into esters (not triglycerides!) through esterification.
Then, we perform transesterification on the triglycerides already present in the oil.