Unit 2 Topic 5a: Lewis Electron-Dot Diagrams
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 7:03 PM on 11/30/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
Lewis dot diagrams
Method of arranging dots representing valence electrons around atomic symbols to stimulate the shape of a molecule
2
New cards
Octet rule
Most atoms are stable with 8 valence electrons and atoms will donate, steal of share electrons to achieve this
3
New cards
Octet rule exceptions
H is stable with 2 electrons
B tends to be more stable with 6 electrons
Non-metals with 3 or more energy levels can sometimes have MORE than 8 valence electrons
4
New cards
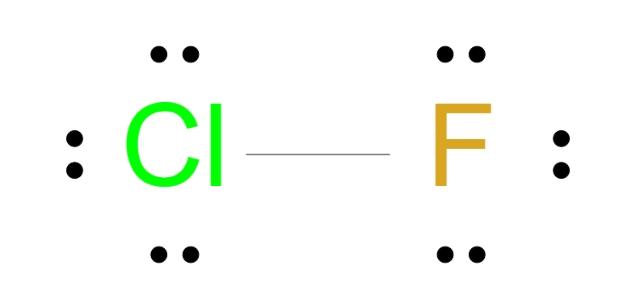
Draw lewis dot diagram of FCl