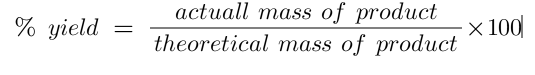3.1.2.5 Balanced equations and associated calculations
0.0(0)
0.0(0)
Card Sorting
1/4
Earn XP
Description and Tags
3.1.2.5 Balanced equations and associated calculations
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
5 Terms
1
New cards
Define atom economy
a measure of what proportion of products become useful products
2
New cards
Equation for atom economy
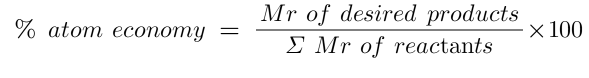
3
New cards
What is meant by the theoretical yield
the mass of the product that should be formed in the chemical reaction - assuming no chemicals are lost
4
New cards
Why is % yield never 100%
reaction is reversable
some of product lost when seperated from the reaction mixture
some of reactants may react in other reactions
5
New cards
equation for percentage yield