history of our planet week 5.1 fire and ice
1/13
Earn XP
Description and Tags
mammal radiation after K-Pg and PETM. This lecture: - Cenozoic climate and mammal diversification - The paleocene-eocene thermal maximum - Methane hydrates and climate change lessons - The origins of us
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
14 Terms
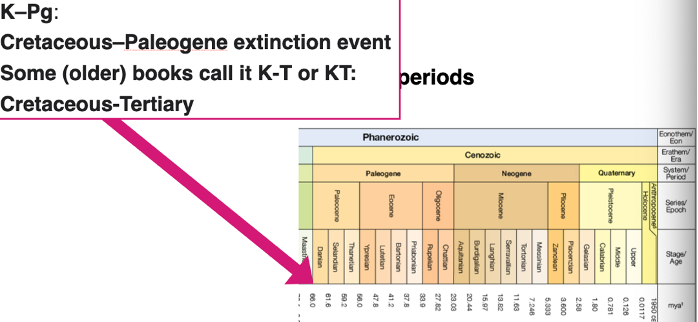
the cenozoic
The latest geologic era
Divided into periods:
Used to be tertiary and quaternary periods
Now Paleogene, Neogene, quaternary
Periods divided into epochs:
E.g. paleocene, Eocene
the cenozoic - mammals
Niches vacated by dinosaurs filled by mammals
Mammals go from 150g to 1kg within 1 Myr
Predominantly diversification within lineages
Remember 66ma = K-Pg – after this evolution and cological diversity happened rapidly
Was only non-avian dinosours extinct
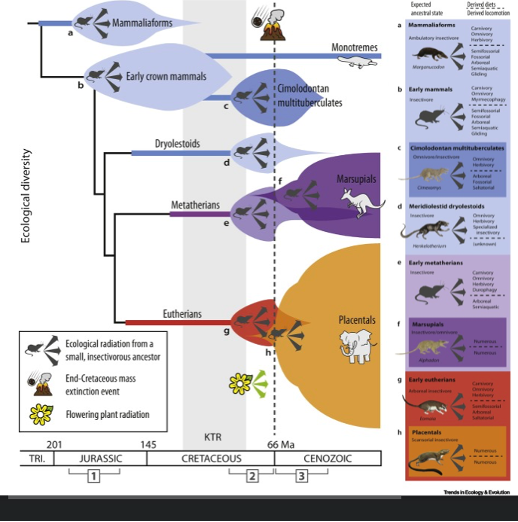
the cenozoic - origin of placental mammal orders
The APP orders:
Even-toed ungulates (artiodactyla):Foregut digesters, Pigs, camels, ruminants
- Odd toed ungulates (perissodactyla): Hindgut fermenters, Horses, zebras, rhinos
- Primates: Lemurs, monkeys, apes
- Led to humans. These things appear quite abruptly
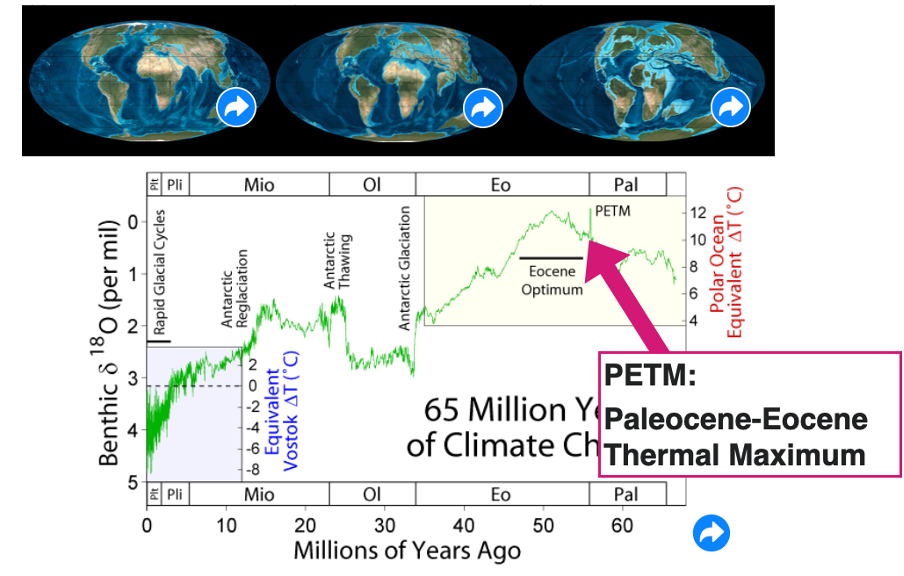
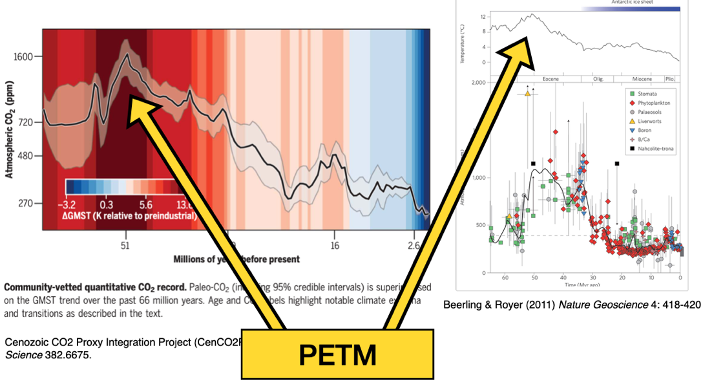
PETM = paleocene-eocene thermal maximum
spike in temp very important
Isotopes of oxygen:
Oxygen 16, Oxygen 18
Oxygen 18 is heavier than oxygen 16
All mass of oxygen is in nucleus
Both of these are stable
Difference in mass can be used to infer what the temp of the earth was e.g.50-6-myr ago
Isotopes of oxygen: Oxygen 16
Protons: 8
Neutrons: 8
Atomic mass: 15.994
Isotopes of oxygen: Oxygen 18
Protons: 8
Neutrons: 10
Atomic mass: 17.999
How is temperature reconstructed:
Foraminifera – pin head single-celled protozoa (tiny organism lives in uppermost portions of the sea) whose shell is made of calcium carbonate (used in bio pump – some carbon used to build shell was previously in mineral form, Prev in air through that carbon silicate cycle). When die, fall out of photic area of sea onto sea floor sediments
Ratio of oxygen isotopes (oxygen 18 and oxygen 16) in their shells is sensitive to temperature and global ice volume
Decreasing temperature gives increasing change oxygen 18 (proportion of 18 O is going up) as increasing ice volume preferentially locks up oxygen 16 in ice sheets
difference
δ18O a measure of the difference between the ratio of 18O to 16O in a ‘sample’, and in today’s oceans
δ18O a measure of how much more 18O there is in a sample, how ‘enriched’ the 18O sample is
seawater is approx:
99.8% 16O
0.2 % 18O
Remember 18oxygen is heavier
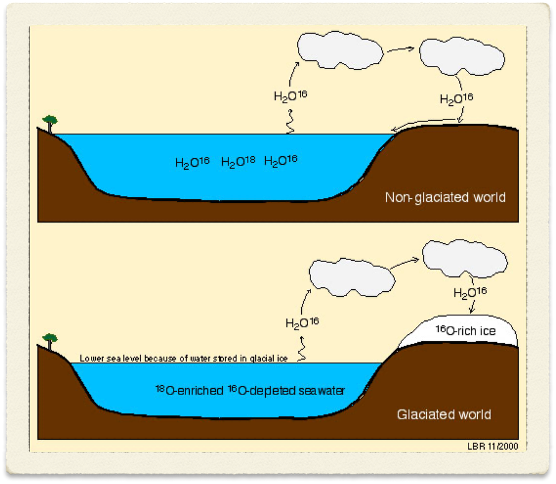
seawater is approx: - in glaciated world
16 O – more is evaporated as it Is most the ocean. Also as it is lighter, it evaporates more easily than 18 O- so the water that comes out of the sea int the clouds has got a different isotopic ratio of 16 to 18 O – so more 16 O gets held up on ice – essentially taking oxygen from sea and putting it onto massive ice sheets
this means depleting ocean store of 16 O
This change in ratio of 16 to 18O means can infer the forams – due to the oxygen it used and the mass
Cenozozoic co2 reconstruction:
The different proxies for CO2 are stating to agree better than they used to
Fossil stomata:
Density on leaf surface increases as CO2 declines (as plant evolves to undertake more gaseous exchange as co2 concentration declines)
Phytoplankton
Carbon isotope fractionation sensitive to CO2
Ancient soils (paleosols)
Carbon isotope fractionation sensitive to CO2e
Paleocene-eocene thermal maximum
Within 20,000 yrs – global temps increased by 5*C
Know it happened due to proxy records
Other things happened at the same time:
Ocean acidification:
Dissolution of carbonate sediments indicates rapid acidification of the ocean (<10,000 years) pH – reduces = more acidic. Roch carbonate sediments and ph. goes down – can chemically react with the carbonate sediments
Requires a large and fairly rapid release of ~2000 GtC into atmosphere to change ocean enough
Prominent clay layer above
Takes more than 10,000 years for carbonate deposition to recover
Carbon cycle perturbation:
Carbon isotope record shows two negative shifts in δ^13C each ~ 1 Kyr long and ~ 20 kyr apart
This requires the release of ^13C- depleted carbon from organic matter or methane
Less carbon is required if the source is more ^13C-depleted e.g. methane hydrates