Ch03 Cell Metabolism
1/89
Earn XP
Description and Tags
APK2105C @ UF | Dr. Nguyen | Ch 3 Cell Metablism
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
metabolism
sum total of all chemical reactions that occur in cells
energy metabolism
reactions involved in energy storage and use
anabolism
These are examples of…
amino acids→ protein
glucose → glycogen
catabolism
These are examples of…
glycogen → glucose
protein → amino acids
hydrolysis
Identify this reaction
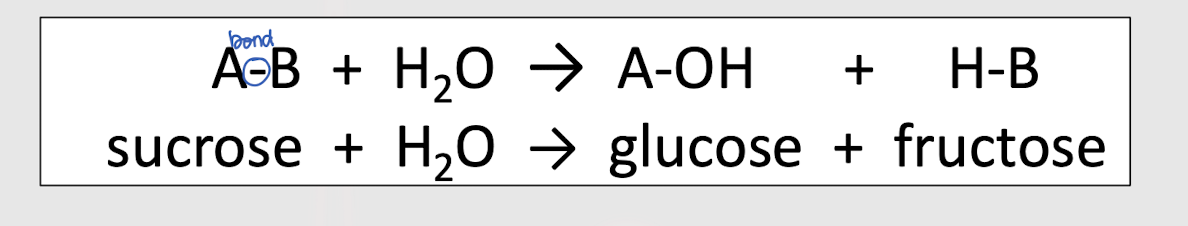
condensation
Identify the reaction
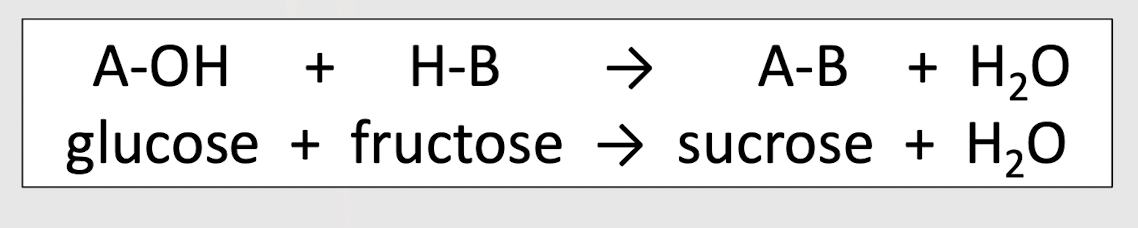
condensation
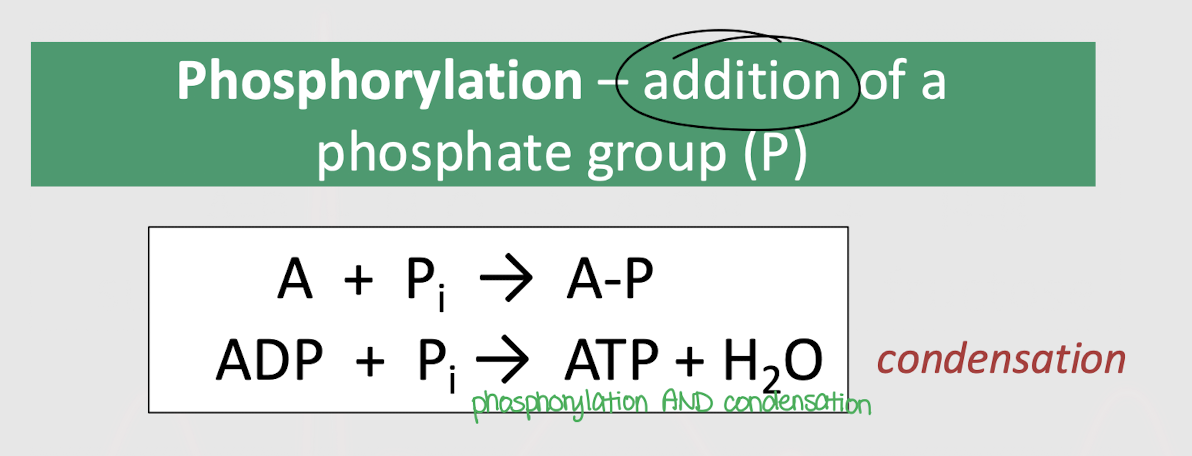
What other reaction accompanies phosphorylation?
hydrolysis
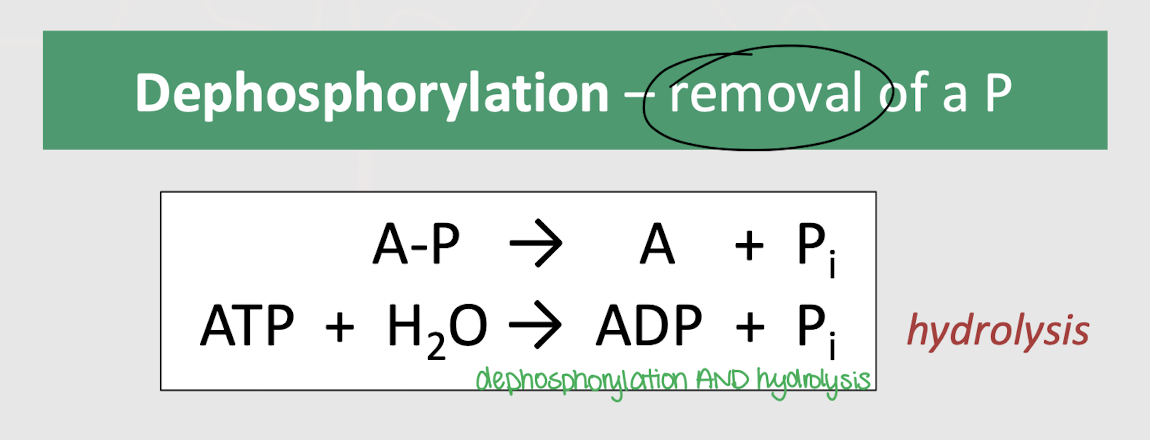
What other reaction accompanies dephosphorylation?
oxidation
removal of electrons; electrons are removed from a reactant, resulting in the formation of an ion product (i.e., charged atoms)
reduction
acceptance/addition of electrons; electrons combine w/ ions to form uncharged atoms
reducing equivalents
H atoms (not ions) are commonly referred to as…
oxidation
When H atoms are removed, is this an oxidation or reduction reaction?
energy
the capacity to perform work
work
The following are examples of _____ in the body:
movement
urine production
cellular repair and reproduction
exocytosis of neurotransmitters from axons
metabolic
All of the work in our bodies is driven by ________ reactions.
kinetic energy
energy associated w/ matter in motion
potential energy
energy stored in matter that can become kinetic energy
first law of thermodynamics
law that states energy can neither be created nor destroyed, just converted from one form to another
second law of thermodynamics
law that states natural processes tend to proceed in the direction that spreads energy
releases; more
If a reaction ________ energy, it’s because the reactant molecules had _____ energy than the products
direction
∆E determines the _______ of a reaction
product; reactant
∆E = E??? - E???
exergonic reaction
Are these characteristics of an endergonic or exergonic reaction?
proceeds spontaneously
∆E = negative
releases energy in the form of work or heat
endergonic reaction
Are these characteristics of an endergonic or exergonic reaction?
does not proceed spontaneously
∆E = positive
energy is used/added
exergonic-endergonic coupling
Energy released from catabolic reactions is used to drive anabolic reactions. This is known as…
calorie
amount of energy/heat necessary to raise the temperature of 1 gram of water 1º C
equilibrium
occurs when the reactant is converted into a product at the same rate that a product is converted to a reactant; ∆E = 0
concentration; large
If the energy difference b/t the reactants and products is large, then the _______ difference at equilibrium will be _______.
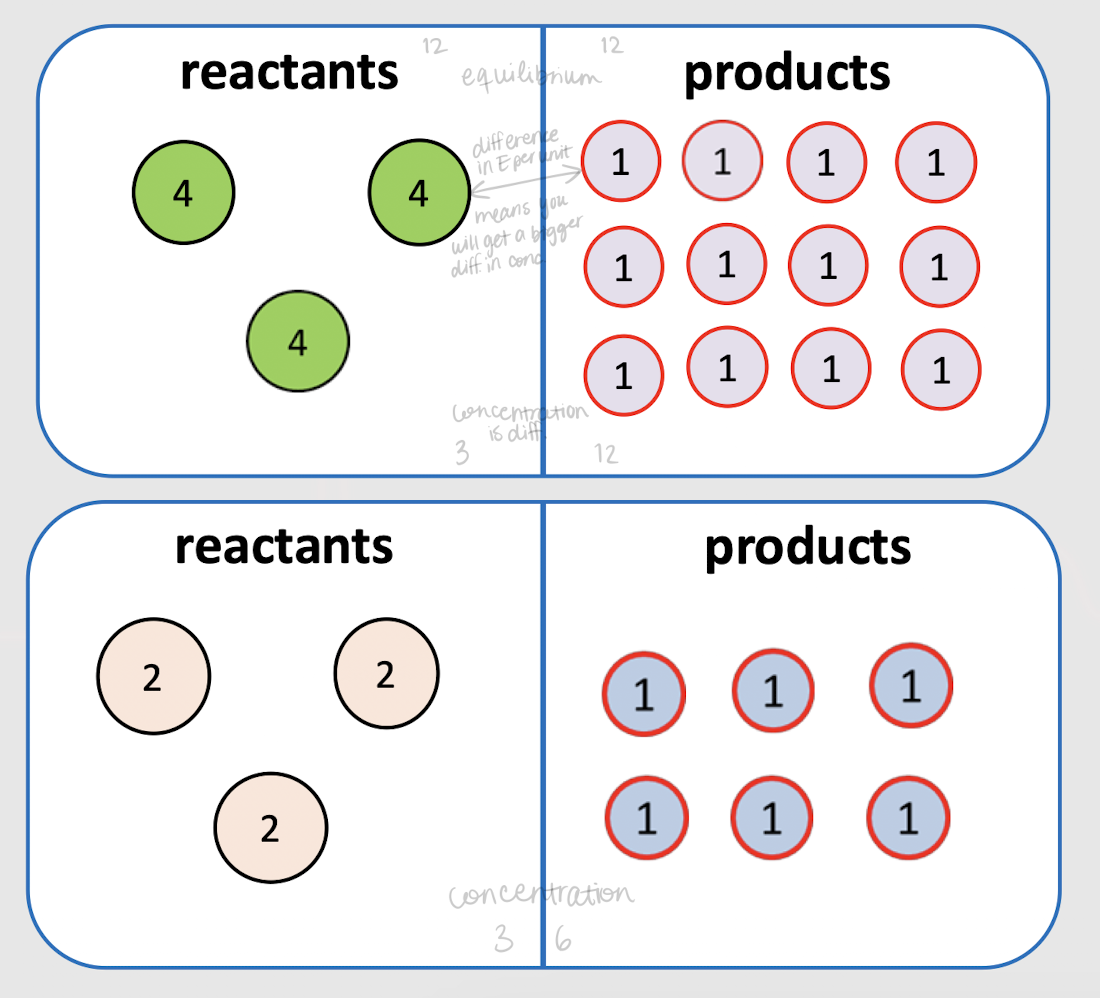
greater; greater concentration
If the energy per mole of the reactants (kcal/mole) is _______ than that of the products, at equilibrium there will be a _______ ________ of products.
law of mass action
law that states than an increase in [reactants] relative to [products] tends to push a reaction forward, and that an increase in [products] relative to [reactants] tends to push a reaction in reverse
equilibrium constant
mathematically describes the relationship b/t [products] and [reactants] at equilibrium; K = [product] / [reactant]
equal; equilibrium
if K=1, there is an ______ concentration of products and reactants at ___________
twice; product; reactant; less; exergonic
If K=2, there is ______ as many _________ molecules at equilibrium as ________ molecules. This means there is _______ energy/mole in the products, so it is an ________ reaction.
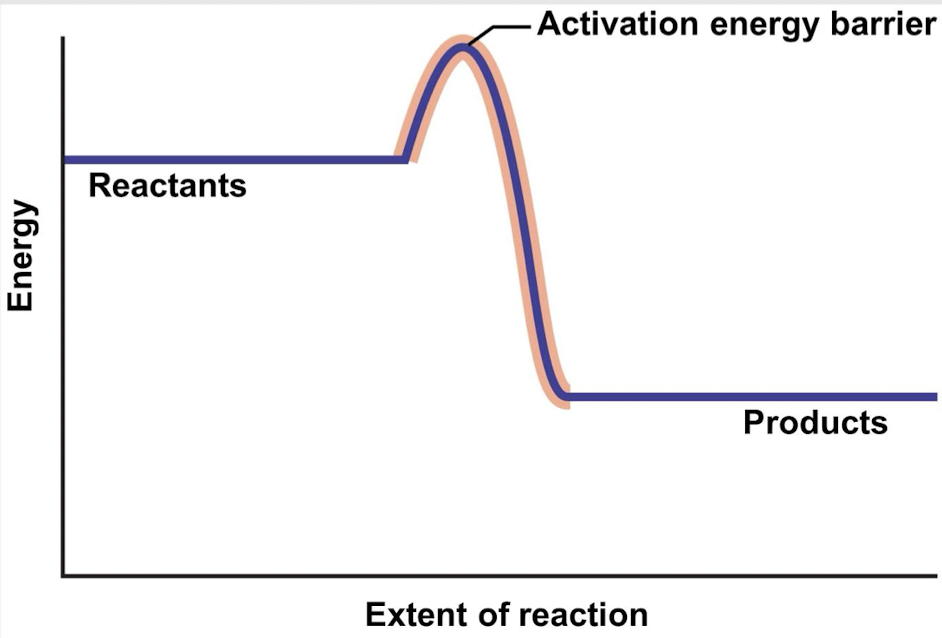
exergonic b/c reactants have more energy
Is this an exergonic or endergonic reaction? Why?
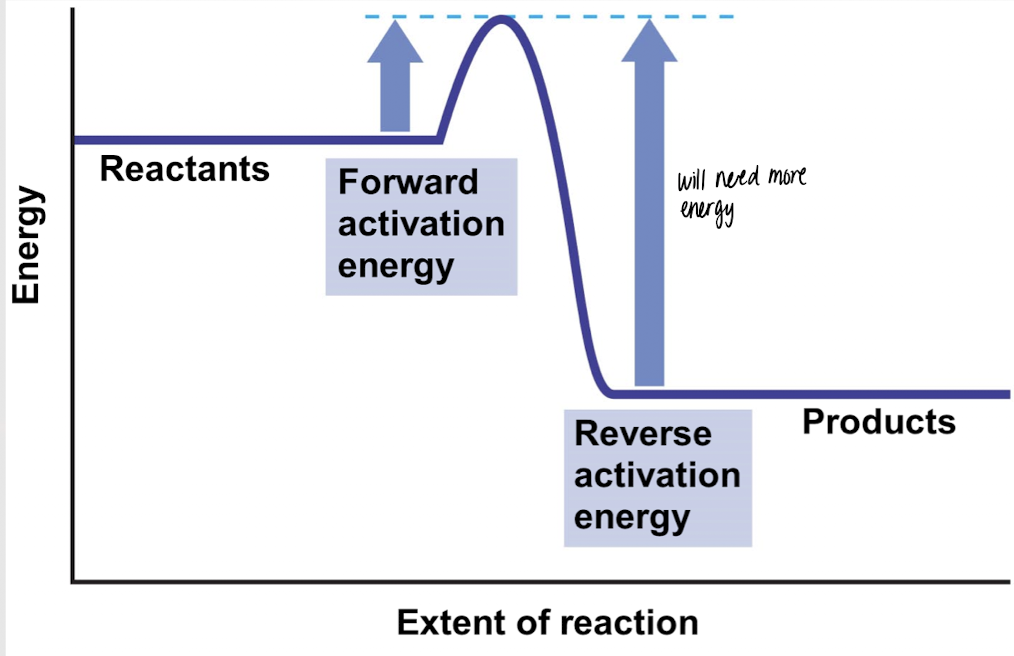
more
Will a reverse activation need more or less energy?
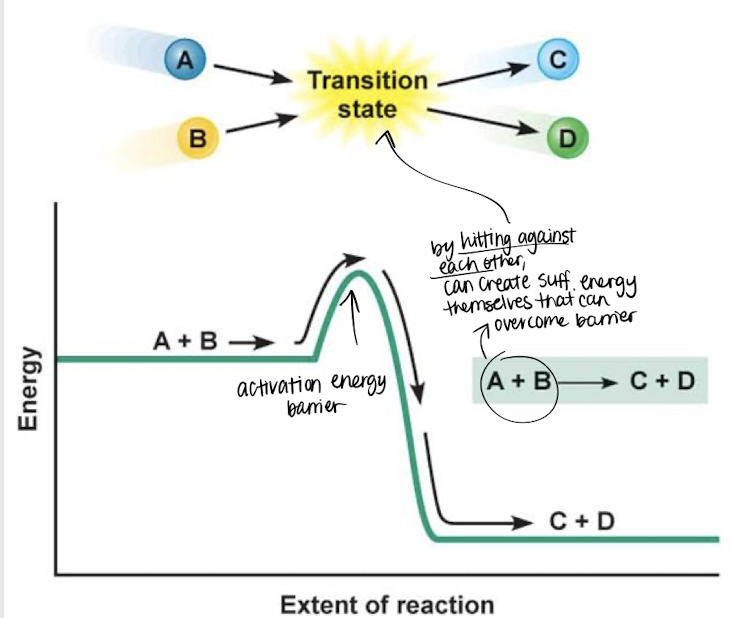
transition state
If reactants A and B collide against each other w/ enough energy, they enter a ________ ______, which surpasses the activation energy barrier, forming products C and D.
cellular demands
How fast reactants are consumed and products are made (reaction rates) must match the _________ _________ of the environment at any given moment.
reactant; product; temperature; height, enzymes
Factors affecting reaction rates:
[?] and [?]
________
________ of the activation energy barrier
________
enzymes
biological catalysts that speed up chemical reactions by lowering the activation energy
do not change the nature of the reaction or the end product
not changed by the reaction
lock and key model
substrate specificity model stating that the shape of the substrate complements the active site of the enzyme
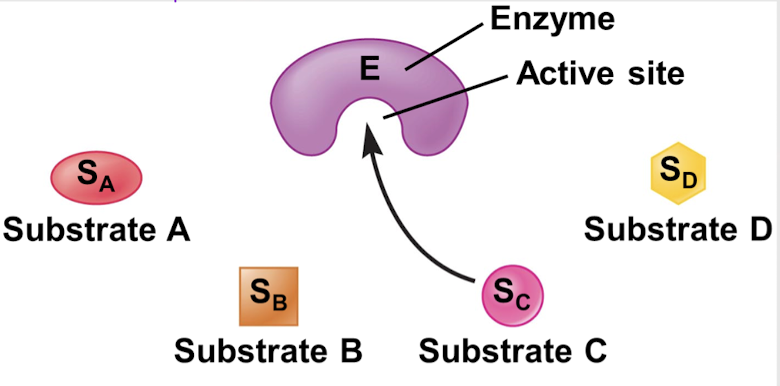
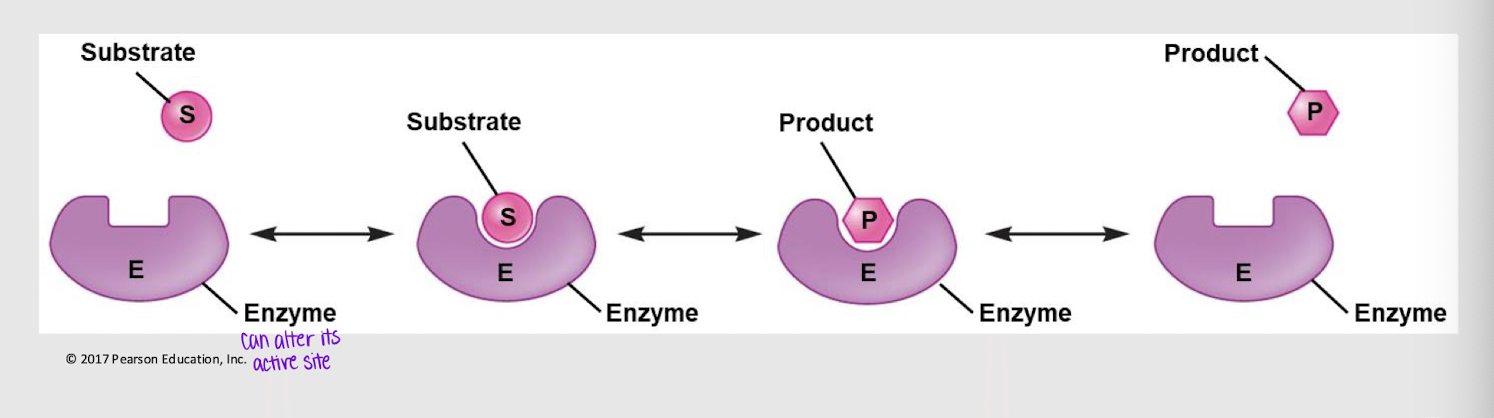
induced fit model
substrate specificity model stating that both the substrate and the product can bind to the active site, allowing the action to be reversible
temperature
Affects rate of enzyme-catalyzed reactions. However, it is tightly regulated, so changes in it are rarely significant.
pH; increasing; structural; charge
Affects rate of enzyme-catalyzed reactions. _______ acidity (decreasing pH) decreases enzyme activity by causing _______ (conformational) changes, as well as altering the _______ at the active site.
cofactors
Affects rate of enzyme-catalyzed reactions. Locks the reacting substance into its active site. W/o it, the reaction can’t take place.
vitamins; minerals; cofactors
Most vitamin deficiency diseases happen by missing _______ and _______ which serve as ________.
coenzymes
Affects rate of enzyme-catalyzed reactions.
Vitamin-derived cofactors.
Function to carry chemical groups from one reaction to another.
Unchanged by reactions → recycled
FAD, NAD, and CoA
What are 3 important metabolic coenzymes?
saturation; faster
Point of [substrate] or [enzyme] that affects rate of enzyme-catalyzed reactions. Higher concentrations of substrates or enzymes result in a ______ reaction rate.
affinity
Affects rate of enzyme-catalyzed reactions.
How tightly substrate molecules bind to their active site.
A lower… means the enzyme is less likely to increase in reaction rate, and vice-versa.
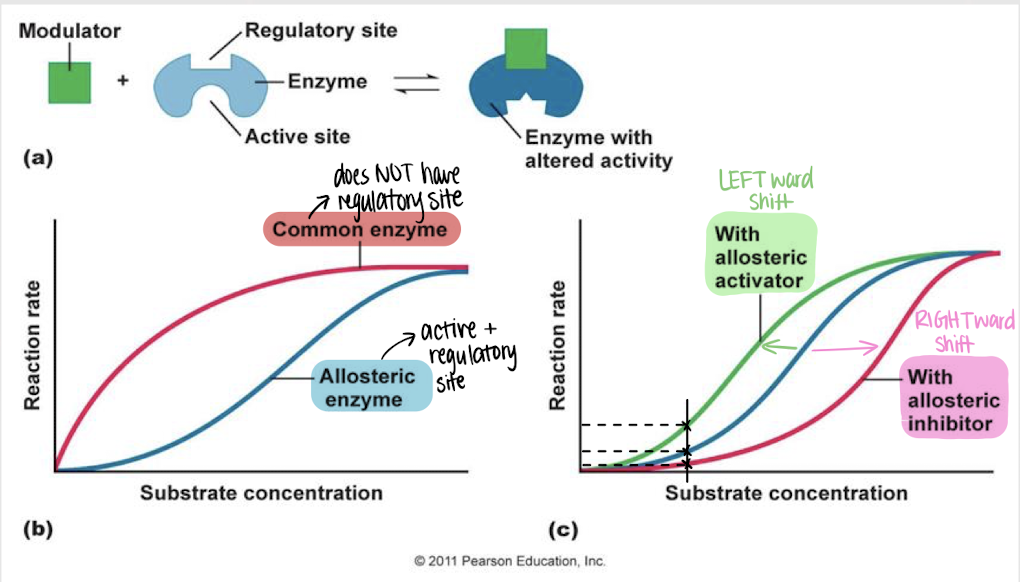
allosteric regulation
enzyme regulation — a modulator binds reversibly to the regulatory site on an enzyme, inducing a change in its conformation and activity
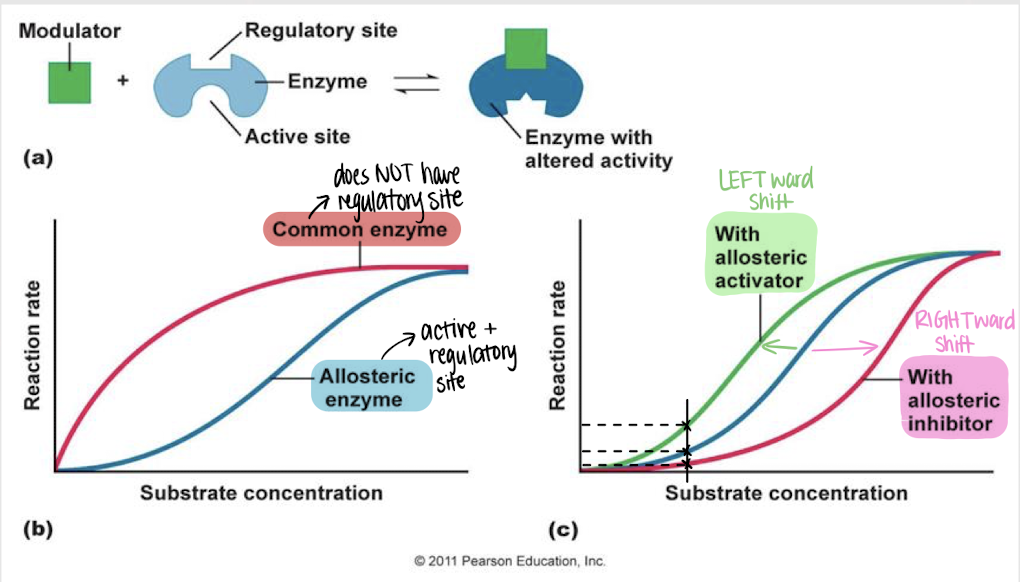
left
Will an allosteric activator cause a left- or rightward shift in reaction rate?
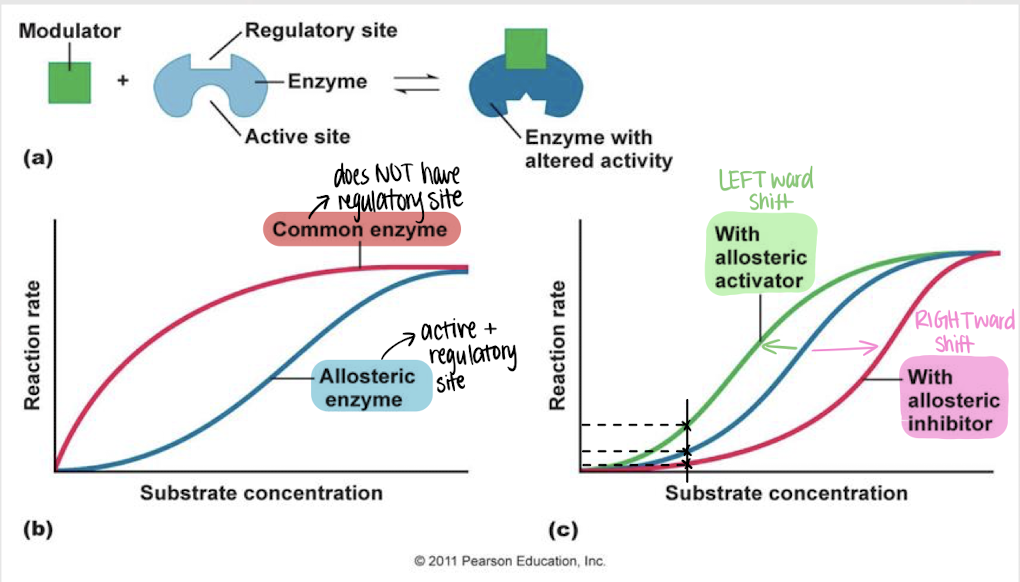
right
Will an allosteric inhibitor cause a left- or rightward shift in reaction rate?
covalent regulation
enzyme regulation — changes in an enzyme’s activity occur as a result of the covalent bonding of a specific chemical group to a site on the enzyme molecule; usually a phosphate group
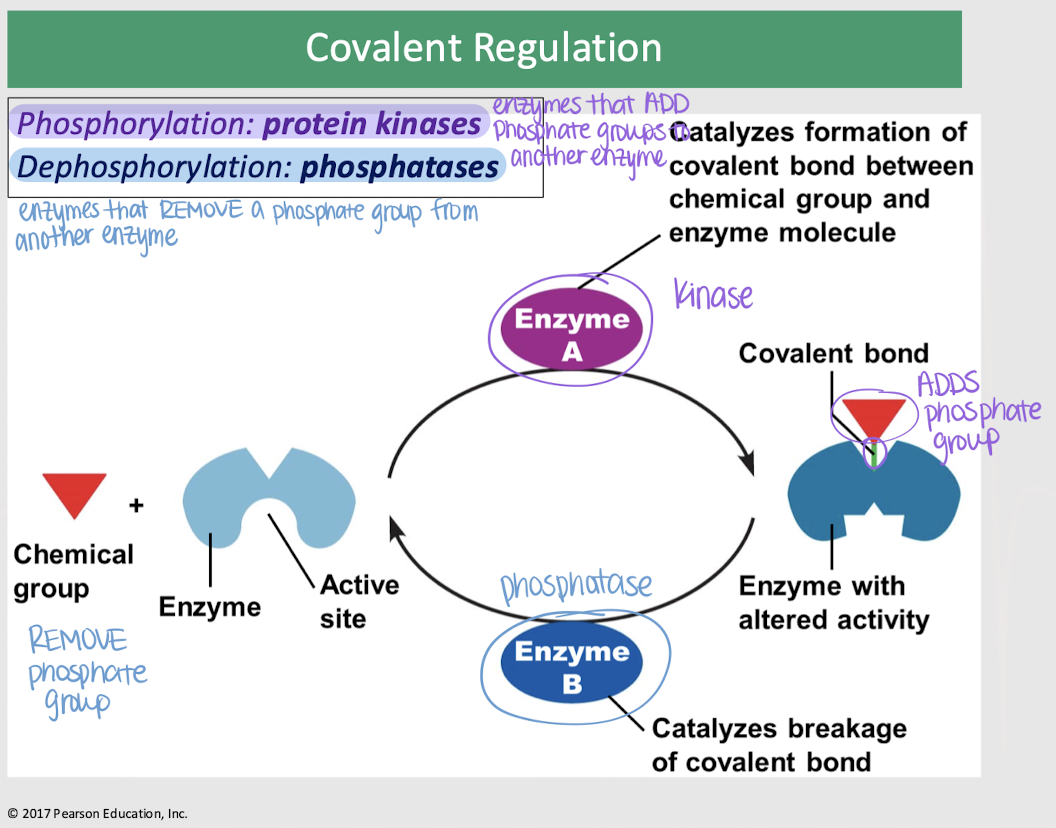
protein kinases
covalent regulation — enzymes that ADD phosphate groups (phosphorylation) to another enzyme, catalyzing the formation of a covalent bond b/t the chemical group and enzyme molecule
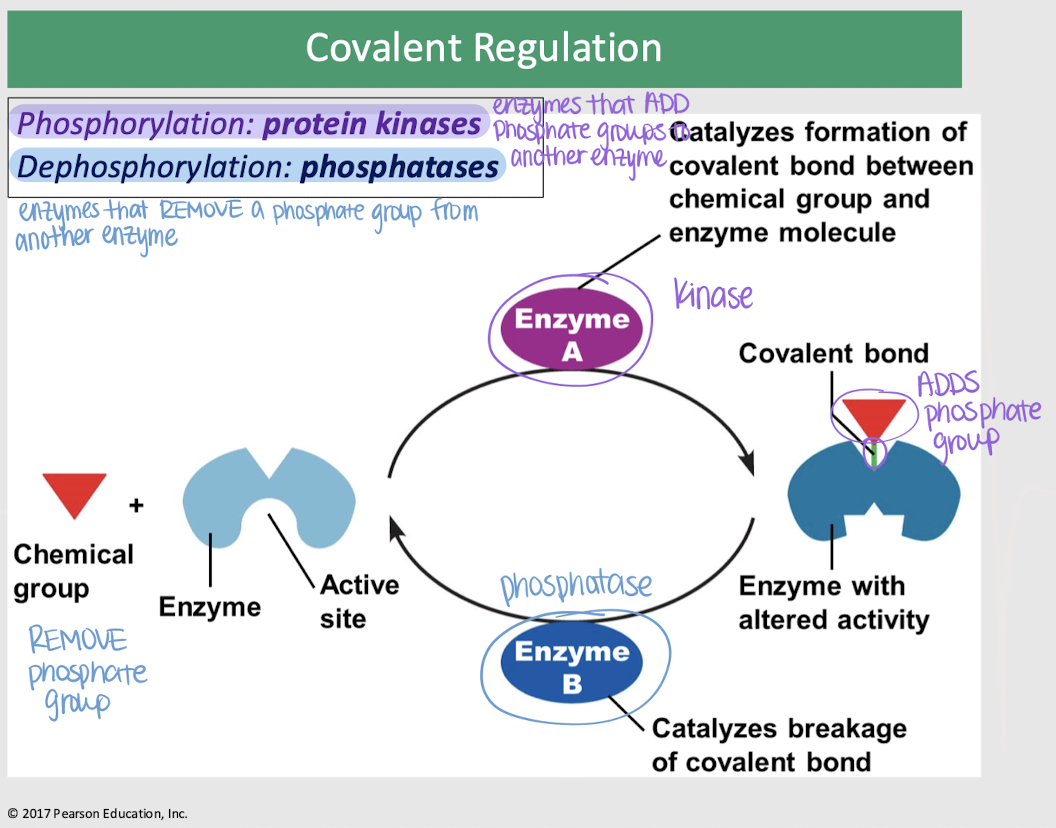
phosphatases
covalent regulation — enzymes that REMOVE a phosphate group (dephosphorylation) from another enzyme
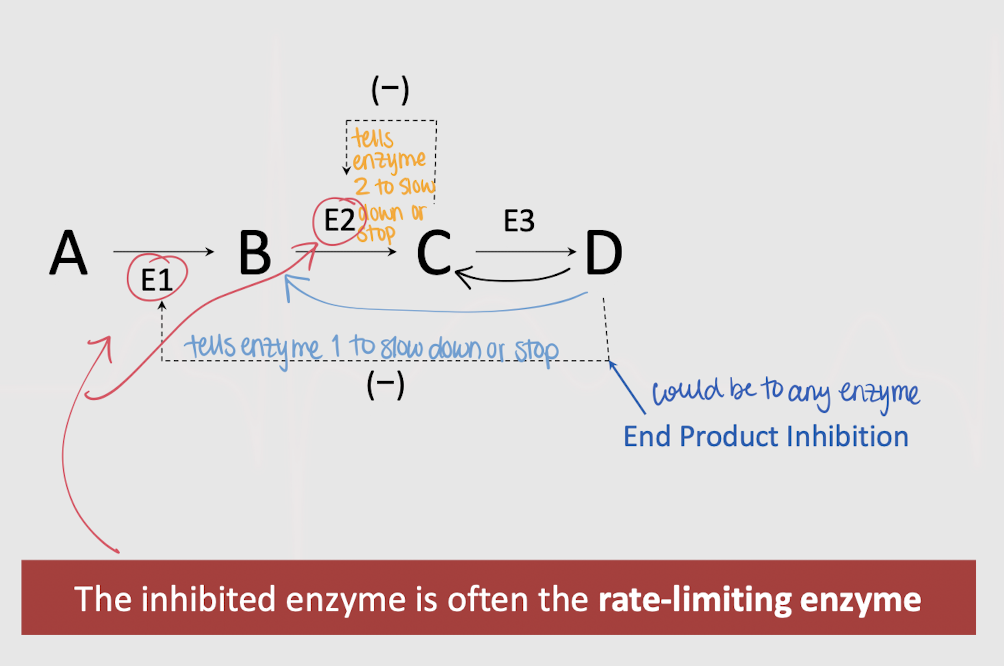
feedback (end-product) inhibition
enzyme regulation — an intermediate product of an metabolic pathway that allosterically inhibits an enzyme that catalyzes an earlier reaction in the same pathway
ATP hydrolysis
process where high-energy phosphate bonds in ATP molecules, containing potential energy, are broken
hydrolyze
When cells need energy to perform work, they must ________ previously formed ATP to obtain energy.
substrate level phosphorylation
mechanism of ATP synthesis where a phosphate group is transferred from a metabolic intermediate (x) to ADP to form ATP; DOES NOT require O2
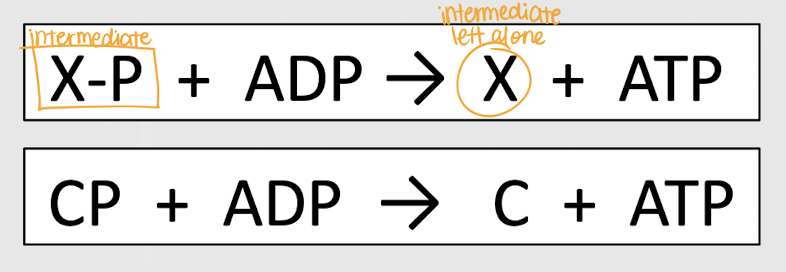
oxidative phosphorylation
mechanism of ATP synthesis where ADP binds w/ an inorganic phosphate by harnessing the energy released when atoms or electrons are transported through the ETC in the inner mitochondrial membrane; REQUIRES O2
6; 6
Glucose oxidation requires 6 O2 molecules and produces how many molecules of CO2 and H2O?
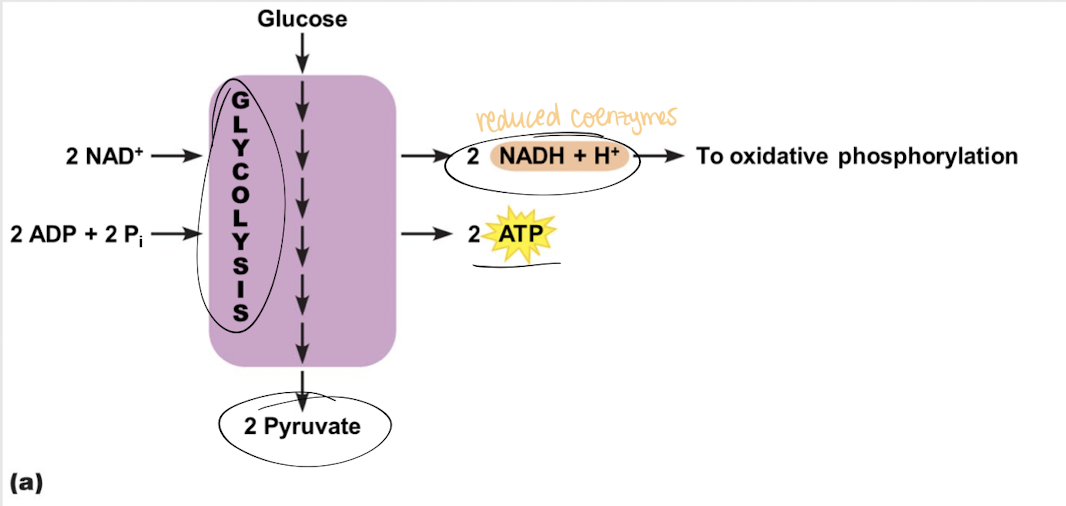
cytosol
Where does glycolysis occur?
mitochondrial matrix
Where does the Krebs Cycle occur?
across inner mitochondrial membrane
Where does oxidative phosphorylation occur?
2; 4; 2; 2
In glycolysis, for EACH molecule of glucose (6-C), how many pyruvate and ATP molecules are produced? How many ATP molecules are consumed? How many NAD+ molecules are reduced to NADH?
anaerobic
Is glycolysis an aerobic or anaerobic reaction?
glycolysis
First step in glucose oxidation where each glucose molecule is broken down into two pyruvate molecules w/ a net gain of 2 ATP and 2 NADH
linking step
process where each pyruvate molecule is converted into acetyl CoA before entering the Krebs cycle, producing NADH and releasing CO2

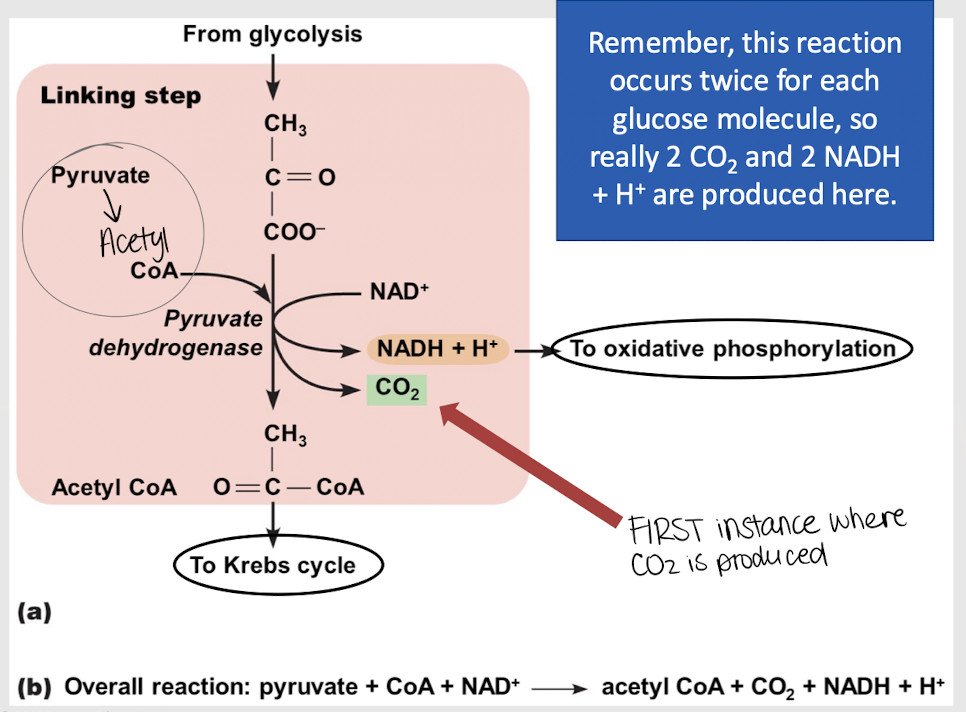
1 NADH and 1 CO2
What does EACH pyruvate molecule produce during the linking step?
linking step
During what step of glucose oxidation is CO2 first produced?
Krebs Cycle
Second step in glucose oxidation in which acetyl CoA is a reactant used to produce CO2 and coenzymes; aka citric acid cycle
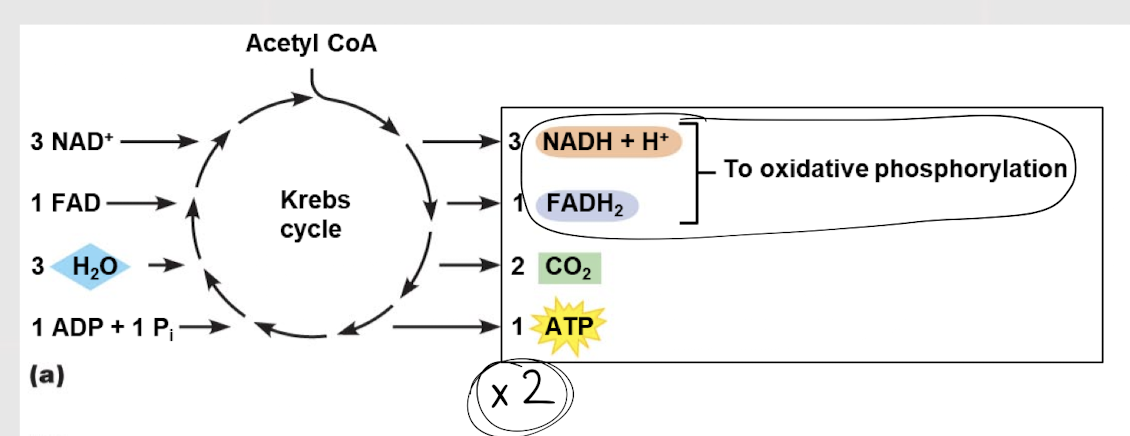
3; 1; 2; 1
For EACH acetyl CoA, how many NADH, FADH, CO2 and ATP are produced?
oxidative phosphorylation
Third step in glucose oxidation where NADH and FADH release electrons to the ETC. As the electrons pass along the chain, ATP molecules are generated.
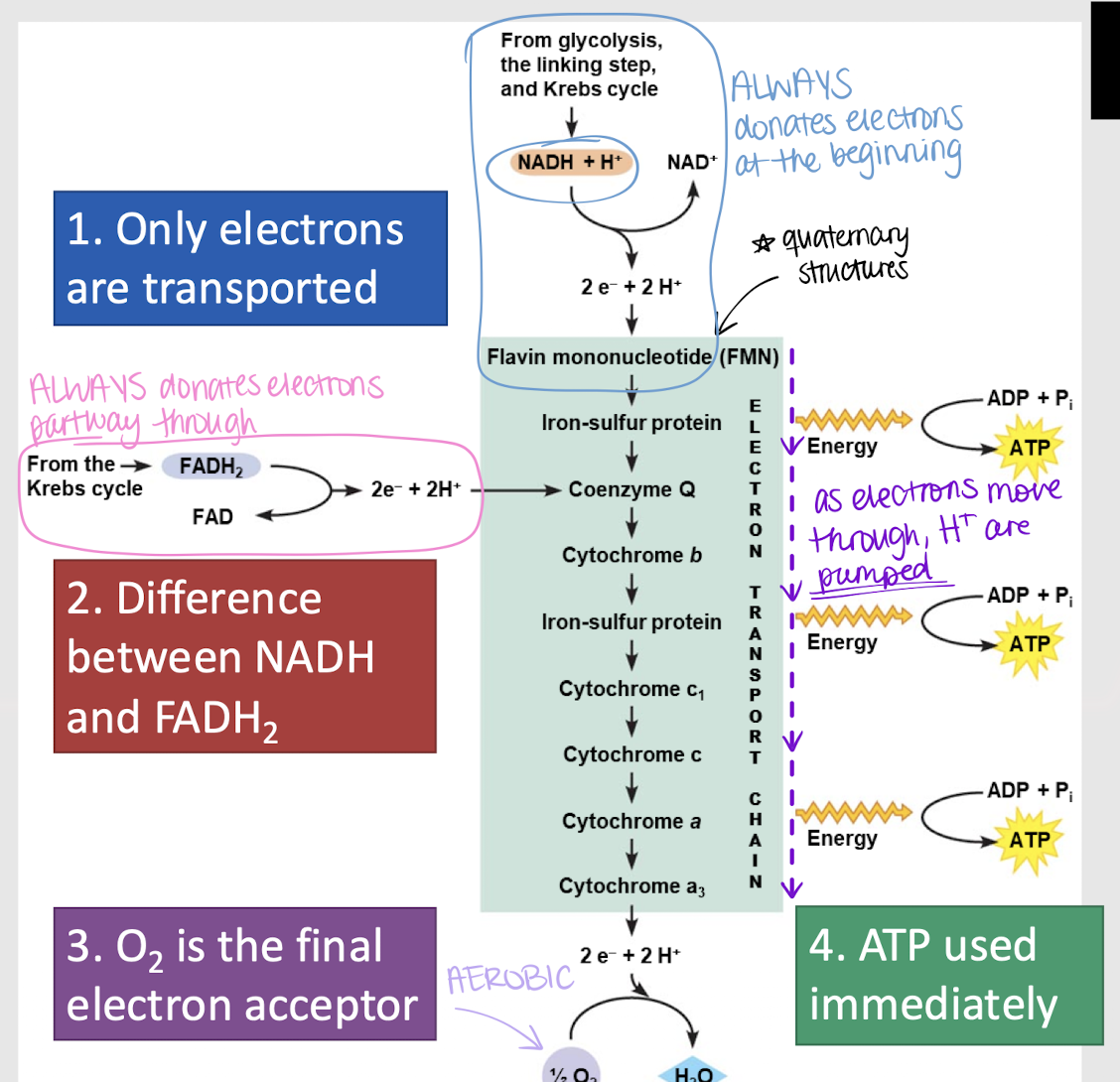
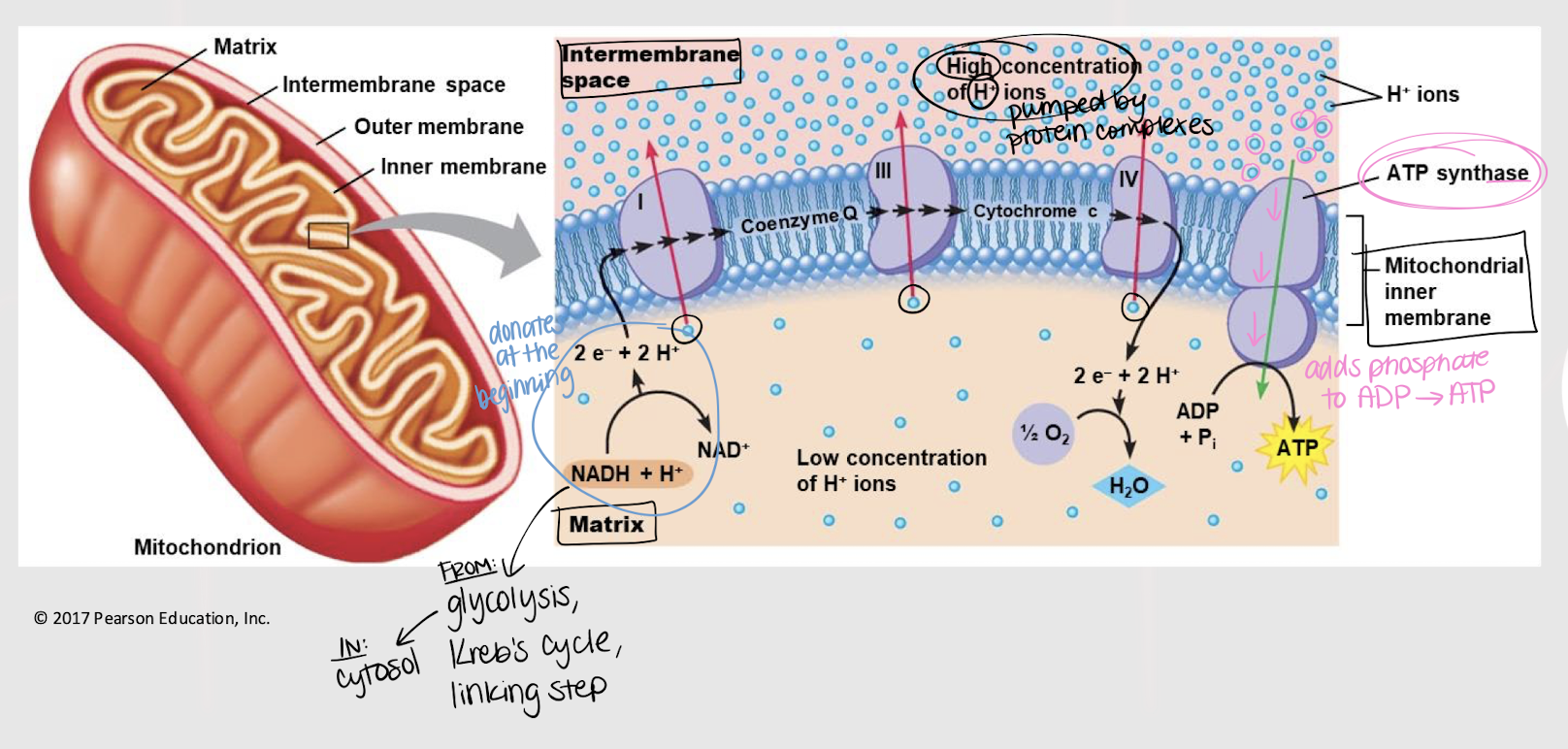
ATP synthase
enzyme that synthesizes ATP from ADP by adding a phosphate group; in the ETC
4
How many H+ ions does the first pump (I) release?
4
How many H+ ions does the second pump (III) release?
2
How many H+ ions does the third pump (IV) release?
4
How many H+ are required to produce 1 ATP?
2.5 ATP; 10/4
What is the average number of ATP produced per NADH molecule? Why?
1.5 ATP; 6/4
What is the average number of ATP produced per FADH molecule? Why?
2; 2
What is the total number of NADH and CO2 produced in the linking step?
6; 2; 4
What is the total number of NADH, FADH, and CO2 produced in the Krebs Cycle?
metabolic demand
the rate at which oxygen must be supplied depends on how fast tissues are consuming it
skeletal and nervous
What are two tissues that are most metabolically active?
slow down; NAD+
If metabolic demand outweighs O2 delivery, the ETC will ____ ____, leaving little to no _____, which is necessary for glycolysis.
lactic acid fermentation
Process that converts glucose into energy w/o using oxygen, producing lactic acid as a byproduct.

cytosol
Where does lactic acid fermentation occur?
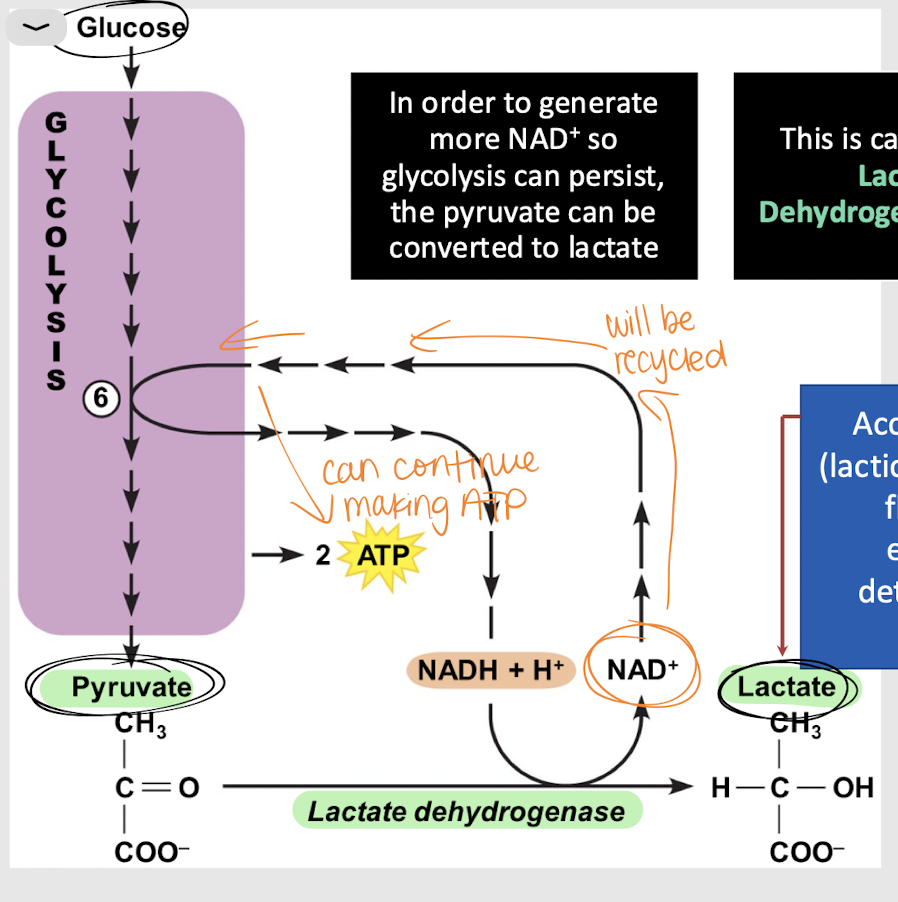
lactate dehydrogenase (LDH)
enzyme that catalyzes the conversion of pyruvate to lactate during anaerobic respiration (lactic acid fermentation)
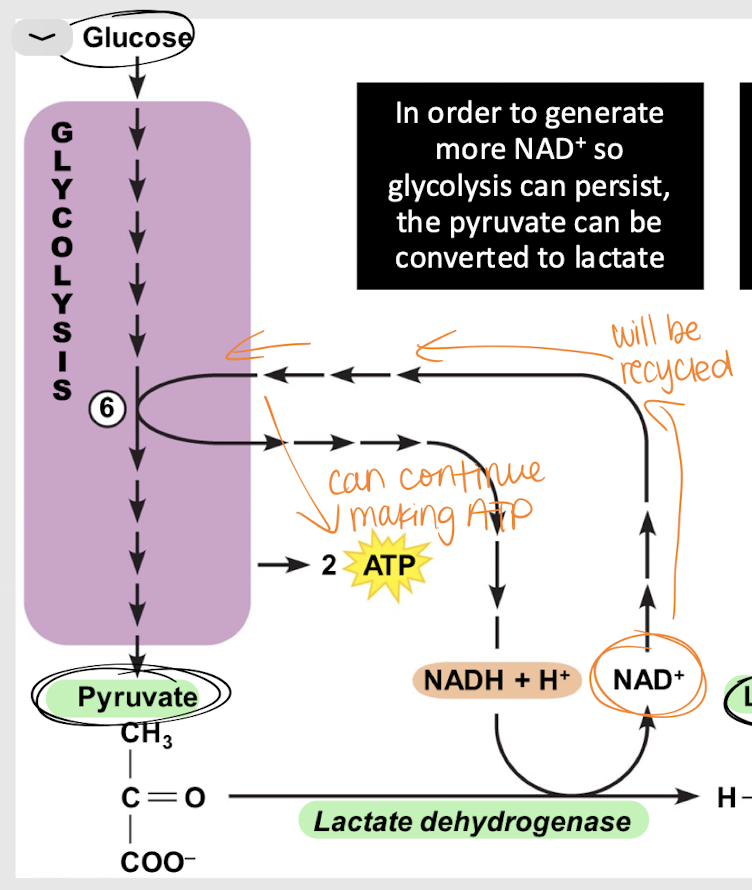
NAD+; ATP
What is the product of lactic acid fermentation? What is it reused for?
interstitial; detrimental
Accumulation of lactate (lactic acid) in the _______ fluid and blood will eventually become _______ for normal cellular functions.