Introduction to Signal Transduction
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
What are the 4 types of Intercellular Cell Signaling
Endocrine signaling (Signal that travels throughout the circulatory system)
Paracrine signaling (cell release signals to another [close])
Autocrine Signaling (Cell release signaling that affects itself)
Contact-dependent signaling
![<ol><li><p>Endocrine signaling (Signal that travels throughout the circulatory system)</p></li><li><p>Paracrine signaling (cell release signals to another [close])</p></li><li><p>Autocrine Signaling (Cell release signaling that affects itself)</p></li><li><p>Contact-dependent signaling </p></li></ol><p></p>](https://knowt-user-attachments.s3.amazonaws.com/a566eea2-777b-4947-ade3-fb9181db1d01.png)
What is Intracellular Signal Transduction
The Process by which a signal produces intracellular biochemical alterations that in turn modify the cell
What is the classic Signal transduction pathway that we have talked about
Stimulus binds to a receptor (internal or external)
This may cause a signal transduction enzymatic reaction
This either causes change in regulation of gene expression OR gene expression-independent change
Both will lead to some changes in cellular function
What is a domain?
What is a catalytic domain?
What is a interaction domain?
A Domain is a sequence of a.a that folds independently and retain their function when expressed in isolation
A catalytic domain tend to be some proteins that have some sort of enzymatic function
Interaction domains: Are selections of a protein that drive proteins into multi-protein complex (Think where the protein-protein interaction will occur).
Why are protein-protein interactions essential for cellular signaling (2)
Recruit and confine signaling protein in appropriate subcellular location
Determine the specificity with which enzymes interact with their target
What are some examples of following Protein interactions that we went over in class
Phosphotyrosine binding domain (1)
Phospholipid binding domains (1)
Ubiquitin Processes binding domains (2)
Cell Death - Related domains (4)
Phosphotyrosine binding domain (1)
SH2
Phospholipid binding domains (1)
PH
Ubiquitin Processes binding domains (2)
Ring, Hect
Cell Death - Related domains (4)
DD (Death domains), DED (death effector domain), BIR (baculovirus IAP repeat), BH ( (Bcl-2 homology domain)
Describe one Key Characteristic of the SH2 domain. How does this characteristic relate to the protein function
SH2 domain has high affinity phospho tyrosine (pY)-containing peptides. But has low affinity for unphosphorylated protein
This helps to ensure that when a protein is phosphorylated it can go to next steps of signaling path (but when its not phosphorylated it goes back0
What is the key Characteristic of the PH domain. What is function of this protein and where is it localized
PH domain proteins are Characterized as having a well-defined binding site for the phosphates in headgroup of phosphoinositide (Think PIP2)
As there are multiple versions of PI, There are multiple PH domains that react with a specific PI-X protein.
Binding to the phosphoinositide allows it to responds to lipid messengers. Therefore these protein domains are localized to the cellular membrane
What are the DD (Death domain) used for. And what role do they play in that function
What proteins is it present in
DD are motifs that are involved in apoptosis
Various proteins that are involved with apoptosis link together via the DD (Think what brings these protein together). The DD are able to dimerize to other DD which helps recruit proteins to a complex
TNDR superfamily and cytoplasmic proteins
What are involved in DED (Death-Effector Domain) and function
What proteins is it involved with
DED is also involved with cell death
inactive procaspases and proteins that regulate caspase activation
These DED come together via homotypic interaction (two of the same domain interaction with each other) between two DEDs
What is BIR involved in
What is unique about BIR
How does BIR function
BIR involved in apoptosis
That all proteins in the IAP family contain at least one BIR domain
BIR is important to interact with a wide range of proapoptotic factor
What is BH involved with
How many different BH domains are there other function
What are they all unique too
BH is involved with mitochondrial apoptosis
There are 4 different BH, have both anti-apoptotic and proapoptotic
DH domains can all be found to the BCL=2 family
What is the main function of receptors
Ligand recognition is a required but not _______ characteristic of a receptor
What happens when translation occur
The main function of the receptor is to bind and recognize a ligand and translate the information into a cell signal
Ligand recognition is a required but not Sufficient characteristic of a receptor
When translation occurs it implies amplification of the input information
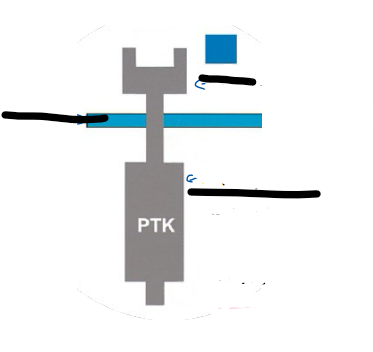
What are the different aspects of the tyrosine kinases receptor (photo and 1 flashcrad)
What are the key role (4)
What is unique about the tyrosine kinase receptor
The tyrosine kinases receptor is a single-pass transmembrane receptor
Cellular growth, differentiation, metabolism and motility
Receptor tyrosine kinase are have intrinsic tyrosine kinase activity
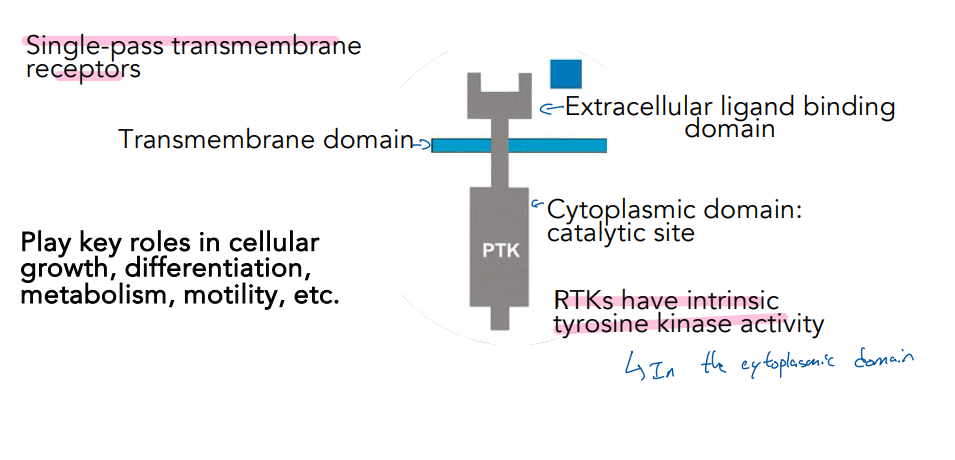
What is a key important concept of the tyrosine kinase receptor that we talked about (related to how many there are)
The extracellular (ligand binding area) domain of the receptor has a large variety of variation; however, the intracellular domain of the receptor (tyrosine kinase area) stays the same.
This allows the tyrosine kinase to recognize a variety of different signaling but have a similar signaling Reponse
How does the tyrosine kinase receptor activated
Once the ligand is bound to the receptor the receptor goes under a conformation change that opens up the kinase domain and allows the active site to become available
True or false
The tyrosine kinase receptor active only triggers 1 signaling pathway
False
Tyrosine kinase receptor can trigger a multitude of different signaling pathways that end up in a change in transcription factors
What are two important domains that all enzymes
Catalytic domain
Interaction domain
What do kinases and phosphates enzymes do
Kinases add an inorganic phosphate from ATP to a target protein
Phosphatases normally remove a inorganic phosphate from a protein
What is ubiquitination, Ubiquitin ligase, DUBs
What are the two function that ubiquitination can regulate
ubiquitination is the addition of ubiquitin to a protein
Ubiquitin ligase is an enzyme that helps transfer a ubiquitin on proteins
DUBs — help remove ubiquitin from proteins
Ubiquitination is important as it regulates NFkB signaling (need to occur), Also important for the internalization of receptors (endocytosis) if the receptor is Ubiqulated
What is the function of GTPase
What activates and deactivates GTPase
the main function of GTPases is that they are molecular switches ( Can either be turn off or on to terminate or start a signaling pathway)
GEFs
GEFs convert GDP → GTP which activates GTPase
GAPs
GAPs convert GTP → GDP inactivating GTPase (Gaps off a phosphate)
What do regulatory proteins contain and what do they not contain
What is the importance of regulator proteins
Regulatory proteins contain interaction domains but not catalytic domain
This allows separation of catalysis and input control into separate genes (redundancy)
What are the 4 possible functions of regulator proteins
Recruit and confine signaling proteins to an appropriate subcellular location
Couple cell surface receptors to intracellular biochemical pathways (bring receptors to interact with protein)
Drive signaling molecules into specific multi-protein complexes
Determine specificity of enzymes with their target
What are the 3 different types of regulator proteins that were mention in class
Docking proteins
Adaptors proteins
Scaffolds proteins
What is the function of Docking Proteins
What are the 2 main aspect of docking proteins that was mention inn class
Docking proteins function as platforms that help recruit signal protein to respond to a stimulate. (Stimulate occurs (conformation change in the receptor that interacts with the docking protein → interacting with the receptor causes the docking protein to have a conformation change that helps it recruit signaling protein)
Docking protein contain a portion of protein
That interact with the membrane (usually made up of; PH domain, myristyl anchor, transmembrane domain)
Intracellular domain that is normally contain SH2 or other domains that help interact with signaling protein
What is the function of adaptor and scaffolding regulatory protein
What is the main different between adaptor and scaffolding protein
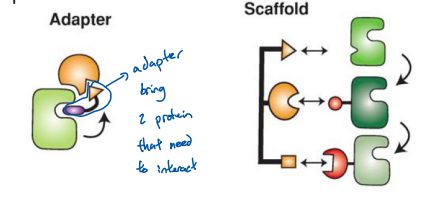
Adaptor and scaffolds recruit specific enzyme or their up stream partner to the same complex (Think is a protein that holds two thing together so their in close proximity for a rxn to occur)
The main difference between adaptor and
Adaptor protein is that adaptor hold 2 things together
Scaffolding holds 3 or more things together
True or False
“Adaptors and scaffolds allow catalytic proteins to play several distinct roles depending on the complex they form”
True
Different complexes can bring different protein to the same enzyme which can cause different downstream affects
What are the two main things that scaffolding proteins can recruit
What can the expression of these protein determine
How can these regulatory function be modified (think what happens when they are post-translated mortified)
The scaffold/adaptor proteins can be either recruit positive or negative regulator protein (either stop are start signaling)
The expression of these protein can determine the function the cell type has (Determine how much of a signal may occur)
The scaffold/adaptor protein can undergo post-translational modification that can either inactivate or active the protein
What is the main point of cell signaling Cross talk
The main point of having multiple pathways that cross talk with each other is to allow intricate control and integration
And enables cells to coordinate multiple signals to ensure the appropriate cellular outcome occurs
What are the two main mechanism of feedback pathways that we talk about in class
Positive feedback
amplify an initial signal
can change the response timing by accelerating or prolonging the time for the signal system to generate a response
Negative Feedback
Inhibit or reduce signaling activity once a certain threshold is reached
Why is it important that we are able to terminal signaling pathways (2)
It is important to maintain homeostasis (return back to a basal state and to prevent over stimulation)
If no termination occurs may lead to the development of a diseases (if can’t stop immune system then have autoimmune disorder)
What are the 4 ways of signal termination that we talked about in class
Termination of plasma membrane receptor signaling
Think phosphorylation/dephosphorylation or internalization of receptors
Synthesis of protein termination signals
NFkB
Degradation of signaling molecules
Enzymatic breakdown of second messengers (like cAMP)
Activation of opposing processes
(Enzymes like GTPase and Phosphates — GAPs turn; GTP→ GDP)