Lecture 3: Telomeres, Cell Aging & Senescence
1/32
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
33 Terms
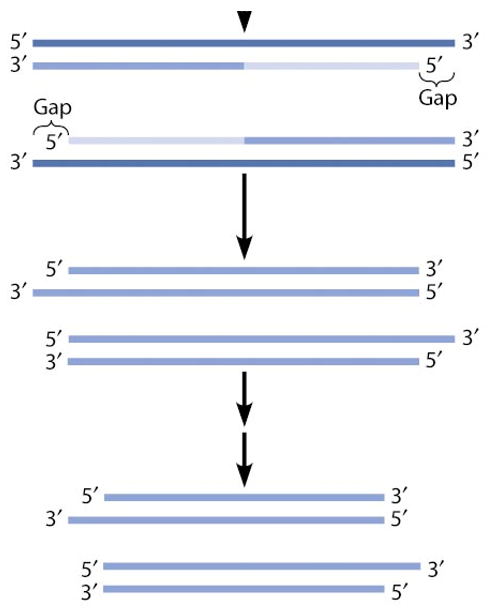
The “End Replication” Problem
Every time the cell divides (DNA replicates), the primers that were laid are not replaced.
Fragments of the DNA will get shorter overtime (20bp lost per cell division)
Can’t remake the entire chromosome on the lagging strand
Human Telomeres
3,000 repeats of the 5’ TTAGGG 3’ sequence
Telomerase
Has two parts:
reverse transcriptase protein (TERT)
rna template with the AAUCCC sequence (TERC); is species-specific

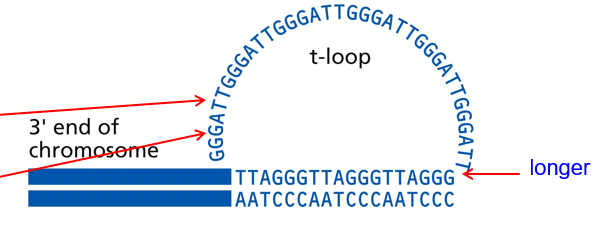
Telomerase in Action
RNA template AAU binds to DNA’s TTA (3’ of the lagging strand), then continues elongation of the telomere
Telomerase makes the 3’ longer than the 5’ end of the antiparallel strand = forms a T-loop (3’ end capping)
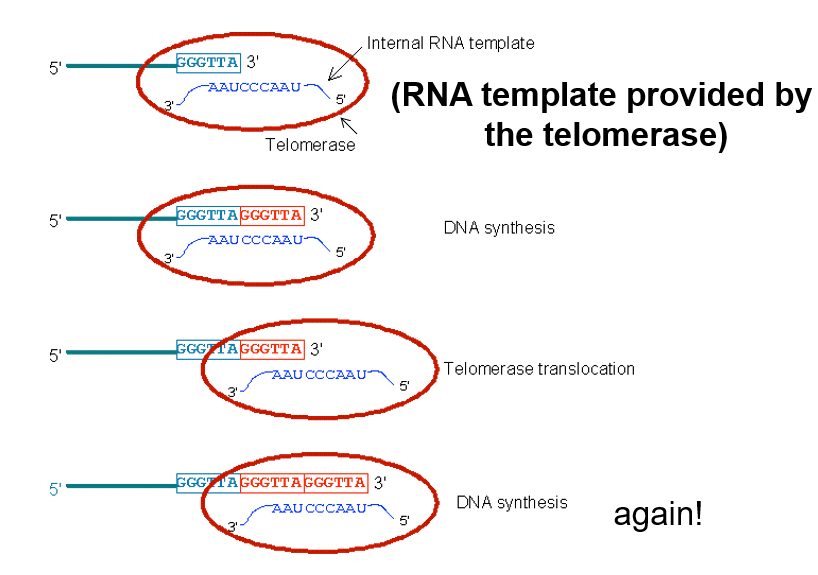
What does a T-loop (3’ end Capping) Typically Represent
A single-stranded DNA
ssDNA is a sign for other molecules to repair DNA
the T-loop is performed to hide the ssDNA and prevent hyper repair
Where are Telomeres Strongly Expressed/On?
In cells that maintain proliferation
stem cells
germ cells
proliferating cells (T cells, intestinal crypt)
cancer cells
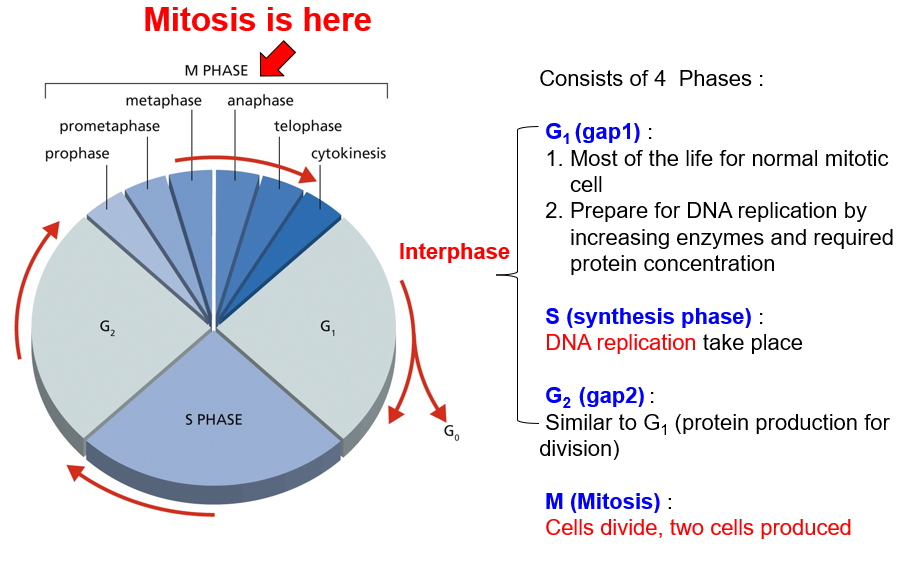
Cell Division
Mitosis: the cell splits into two new daughter cells
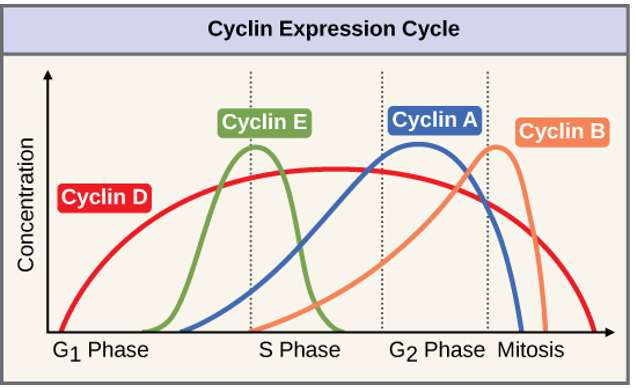
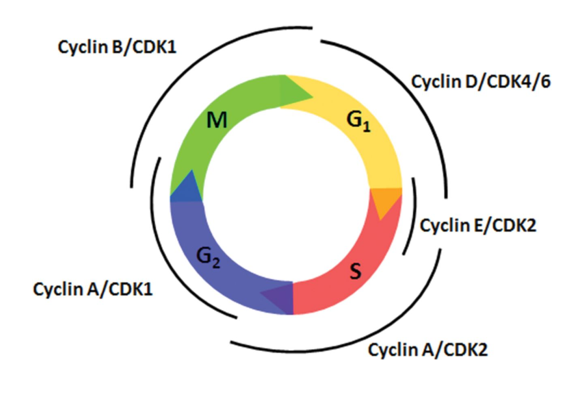
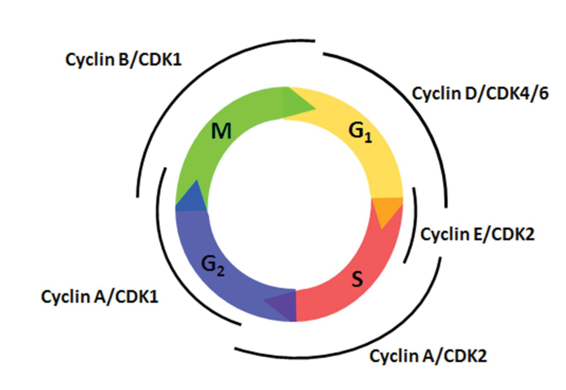
Cyclins
promote progression through the cell cycle
the “license” the stages of the cell cycle
they partner with CDKs (cell division kinases)
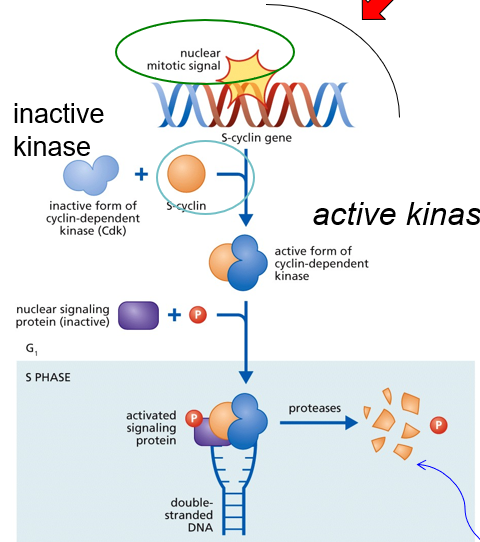
Initiation of DNA Replication
Extracellular Mitotic Signal (i.e growth hormone) expresses S-cyclin
S-cyclin protein binds to CDK to activate it
A nuclear signaling protein (inactive) and phosphorous join the S-cyclin/CDK complex
All four molecules signal the nucleus to start replication
Protease degrade cyclin so that DNA replication occurs only once
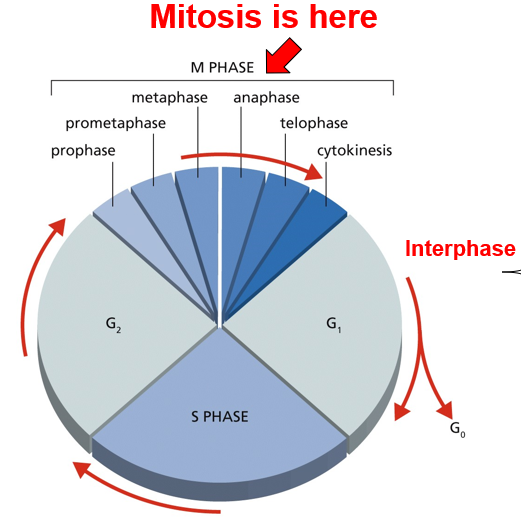
G1 Checkpoint
Checkpoint for DNA Replication
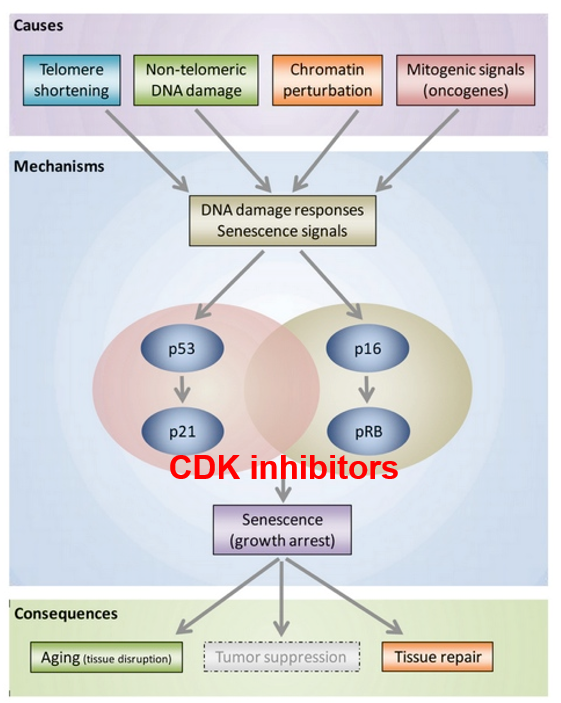
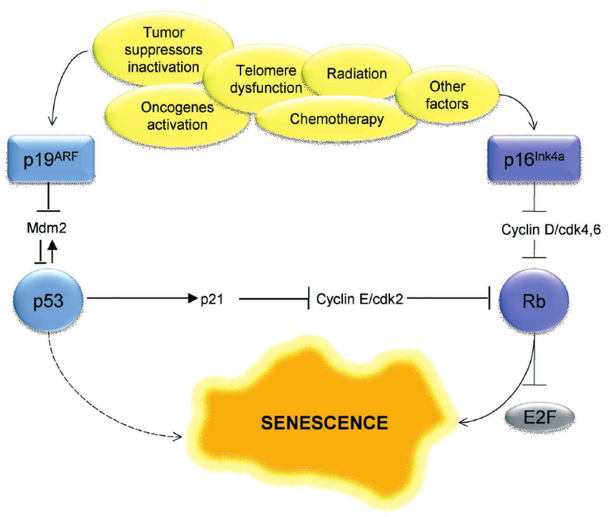
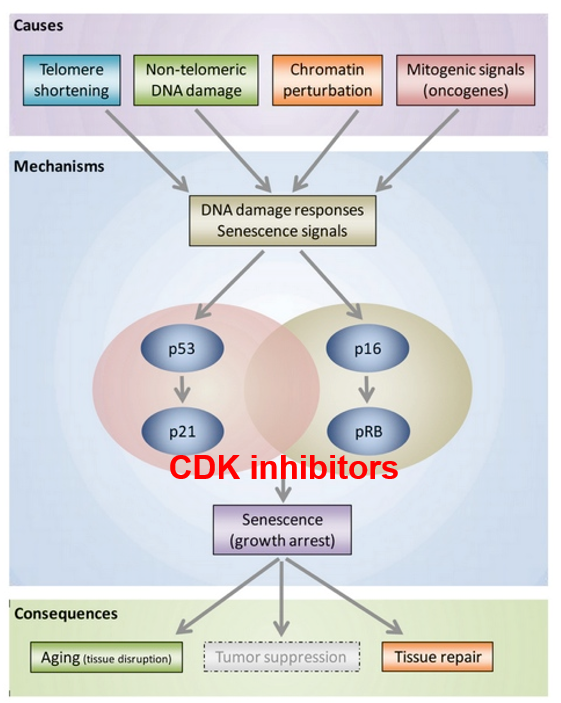
G1 Checkpoint: p53 Pathway
p53 kinase senses DNA damage and sends a signal
DNA needs to be fixed before replicating it
p53 protein expressed p21
p21 inactivates the S-cyclin/CDK complex
p21 gets degraded by protease after DNA is repaired
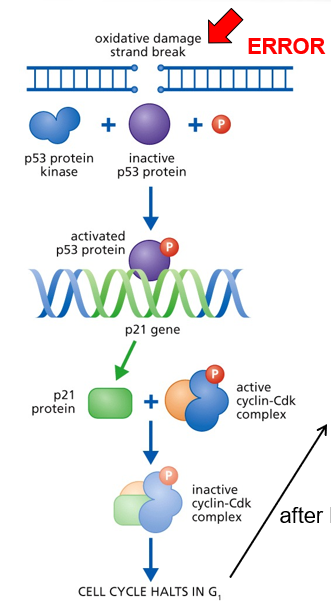
p53
a tumor suppressor
induces cell-cycle arrest for damaged DNA
senescent cells use p53 to promote the arrested cell cycle state (arrest between G1 and S phase)
Hayflick Limit
cells have a limited ability to divide
Replicative Senescence
stops division
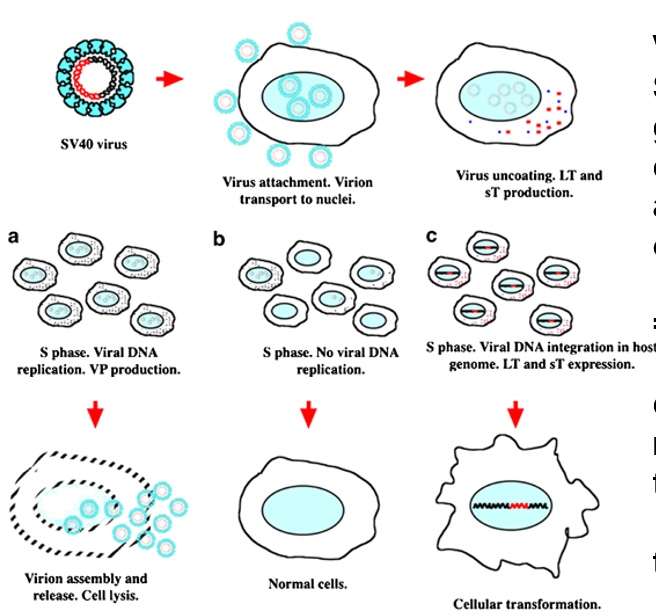
Cell Immortality is Possible in Culture
viral protein SV40T antigen integrating in the gene can lead to indefinite number of cell doublings = transformation
Must be transformed to grow forever
Tumorigenic
How to identify senescent cells
staining with beta-galactosidase (blue)
appear larger and flatter
decreased number of proteins associated with DNA replication, and overall decreased rate of protein synthesis
increase in pro-inflammatory cytokines
Mitotic Clock Theory
old cells sense short telomeres and induce cell cycle arrest
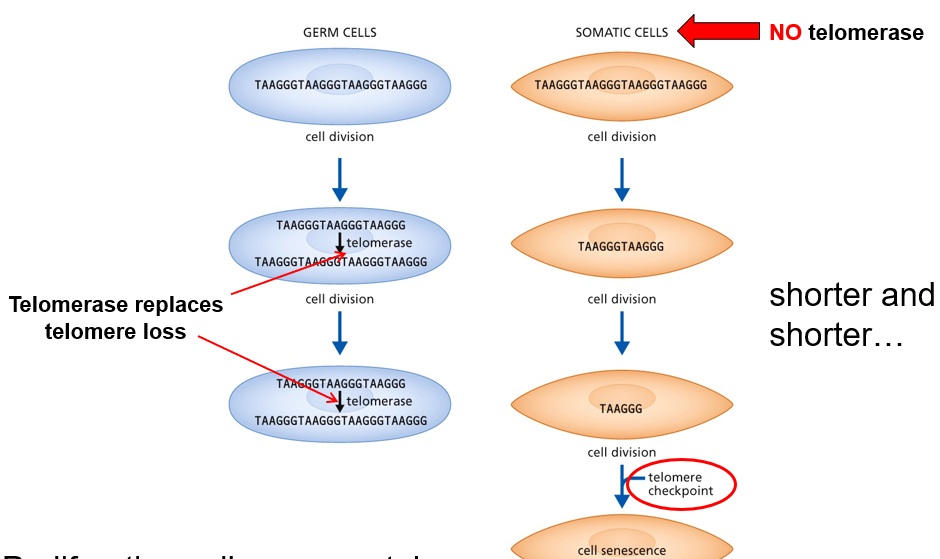
How do shortened telomeres cause somatic cell senescence?
short or uncapped telomere is read as a strand break
p53 is phosphorylated upon stress
activates p21
p21 inhibits the Cyclin E/CDK2 complex
Molecular brakes at G1 (cell cycle arrest)
This results in the cell becoming in a replicative senescence state
How do you start the cell cycle again if p21 made it go into arrest?
a mitogen signal from the outside starts the cell cycle back up again (hormones, steroids, etc)
Maintaining the Senescent State
p53 and pRb pathways are involved
these are tumor suppressor genes
pRb
retinolbastoma
works with p53 to maintain a senescent state for the cell due to telomere shortening
Cyclin/CDK complexes inhibit Rb
Inhibition of Rb (is hypo-phosphorylated) leads to cell cycle arrest
p16
like p53 where it inhibits Cyclin-CDK complexes, specifically Cyclin D-CDK 4,6
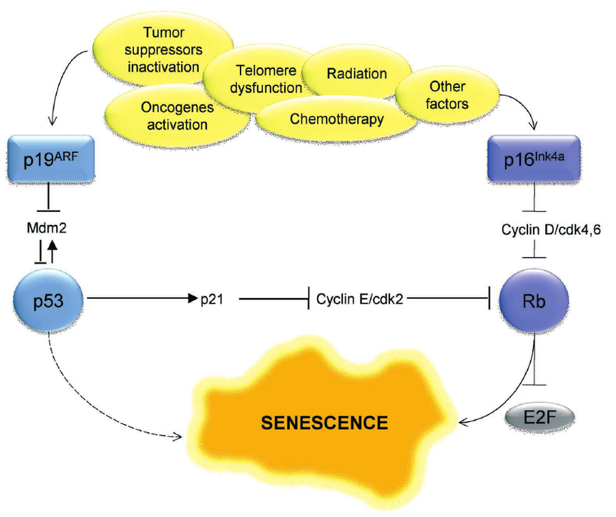
Triggers of Cellular Senescence (Telomere Shortening)
UV radiation
ROS (reactive oxygen species
nutrient imbalance
chromatin changes
oncogene activation
What does beta-gal. activity mean
senescence (blue color)
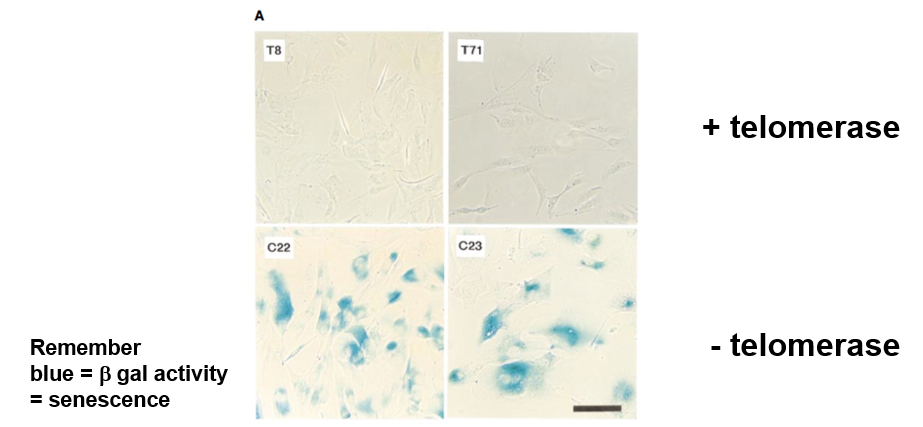
Telomerase Gene
hTRT
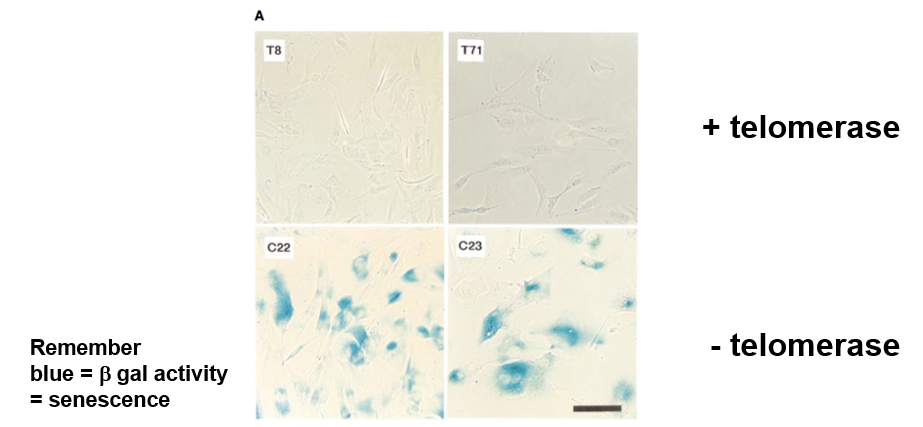
What did hTRT do experimentally
increased the doubling number (changed the Hayflick limit)
increased telomere length
Having long telomeres…
is associated with long life
Dyskeratosis congenita
a progeria (pro- premature + geras- old age)
defects in telomerase
high cancer
short lived (teens-50s)
Cancer & Telomeres
Active telomerase makes cells immortal, leading to a mass of cells (cancer)
What happens if you stop telomerase activity all together
disrupt fertility, wound healing, and the ability to fight infections
What does senescent cells lead to
decreased risk of tumorigenesis
increased inflammation (common feature of all age-related diseases)
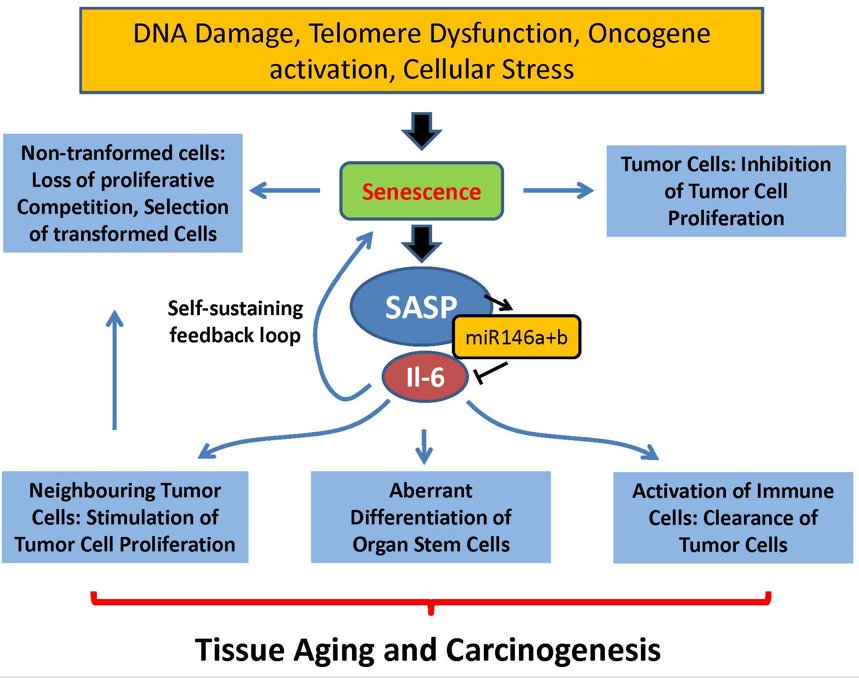
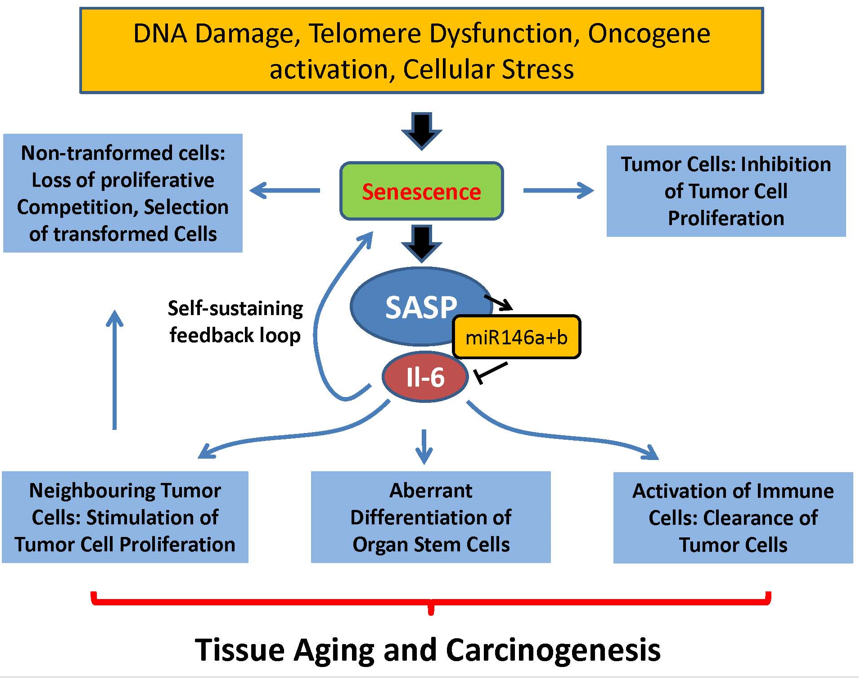
Senescence-Associated Secretory Phenotype (SASP)
senescent cells result in a certain phenotype (SASP)
active senescent cells signaling (senescence messaging secretome, SMS) produce
cytokines
growth factors
proteases
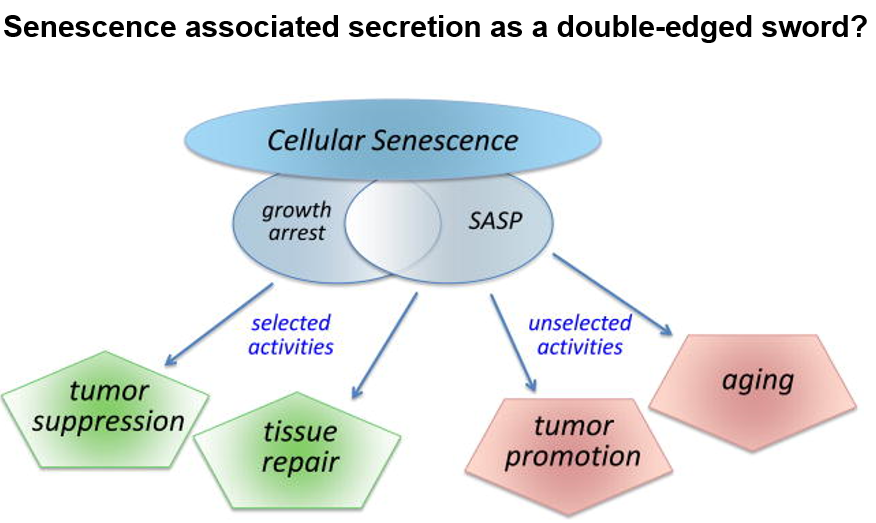
Senolytics
class of drugs that selectively eliminate senescent cells
promote tumor growth
decrease inflammation (decrease risk of age-related diseases)