7-Gas Phase Chemistry
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
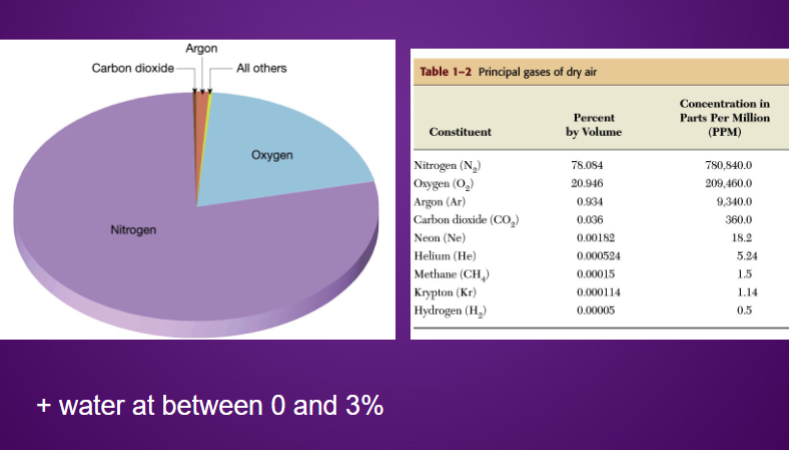
What are the compositions of gases in the environment?
Highly oxidising atmosphere which drives atmospheric chemistry.
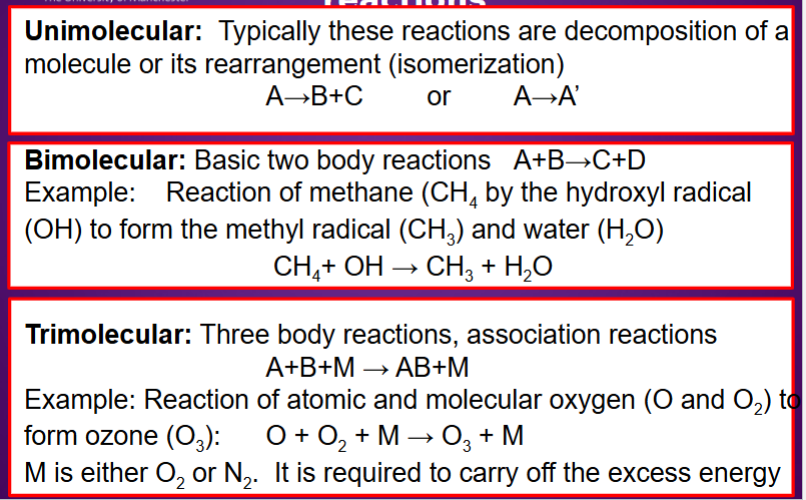
What is unimolecular, biomolecular, and trimolecular reactions?
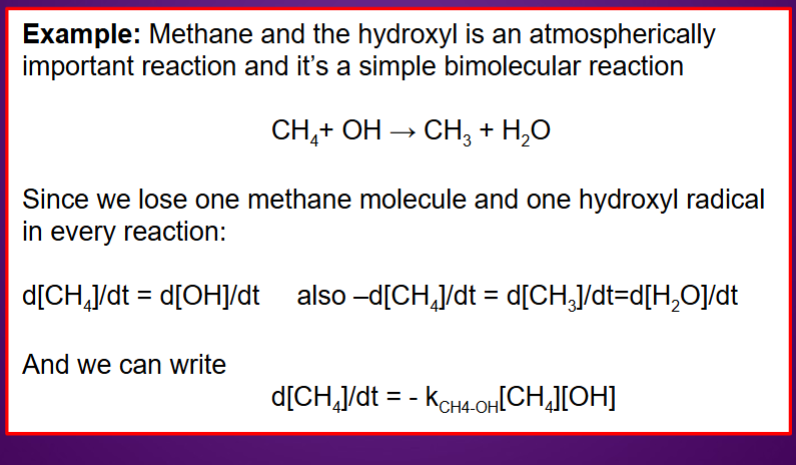
What is the rate of reaction?
Change in concentration per unit time.

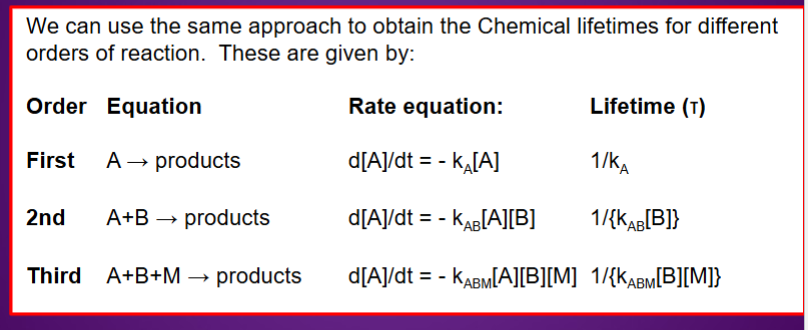
What is meant by first order, second order, third order?

What is chemical lifetime?
Time at which the reactant falls to 1/e of its original value
What is photolysis?
Decomposition of molecules by the action of light (breaking of bonds within molecules)
How do we use photolysis rate with rate of change of concentration?
Each rate for equations is different as takes different amount of energy to break molecule, J is different for each reaction

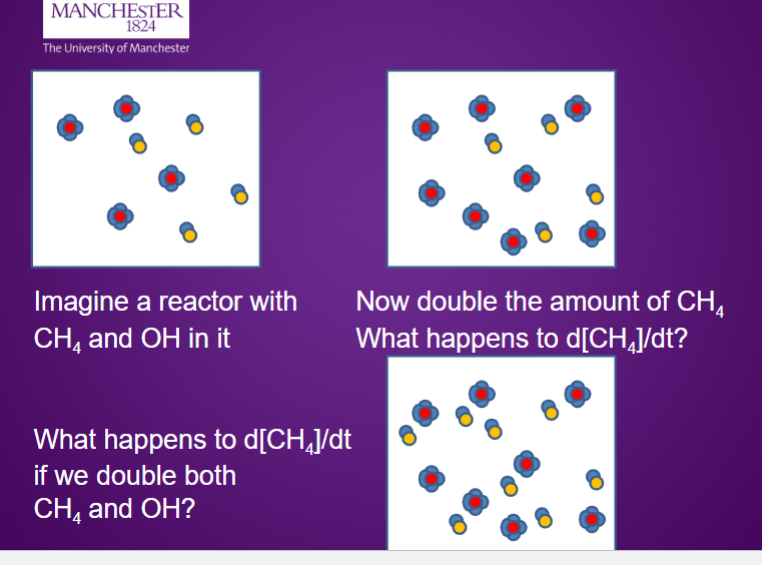
Doubles
x4
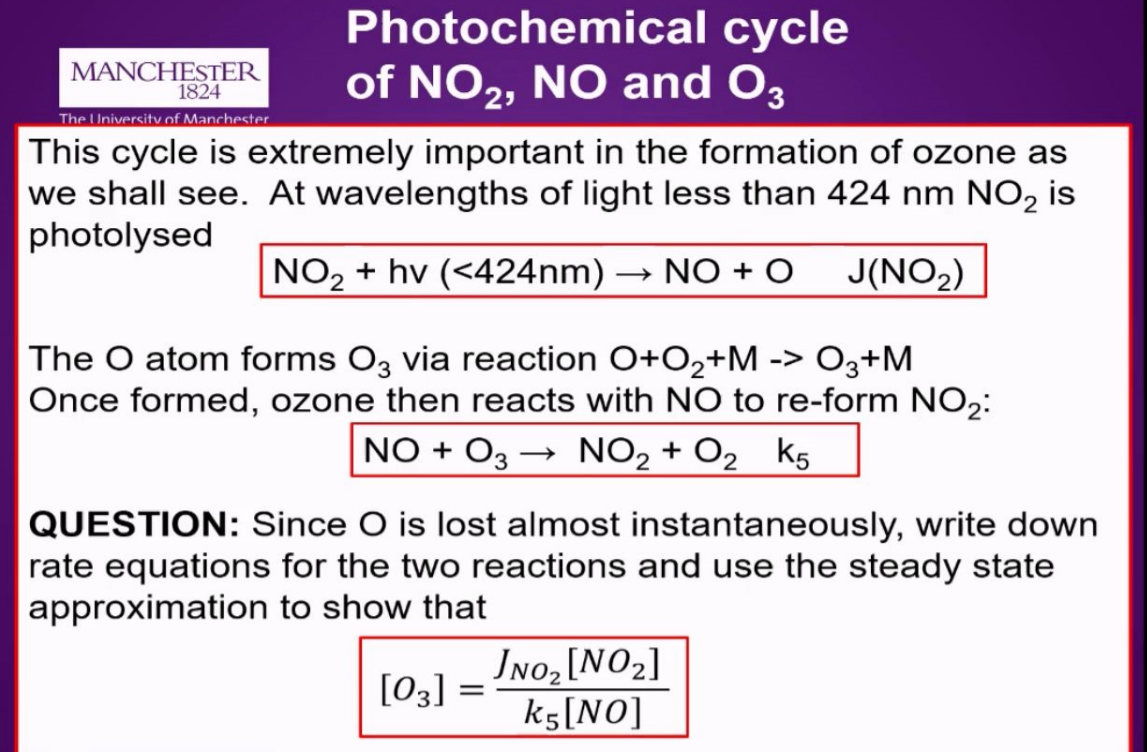
What is the No2, NO, O3 photochemical cycle?
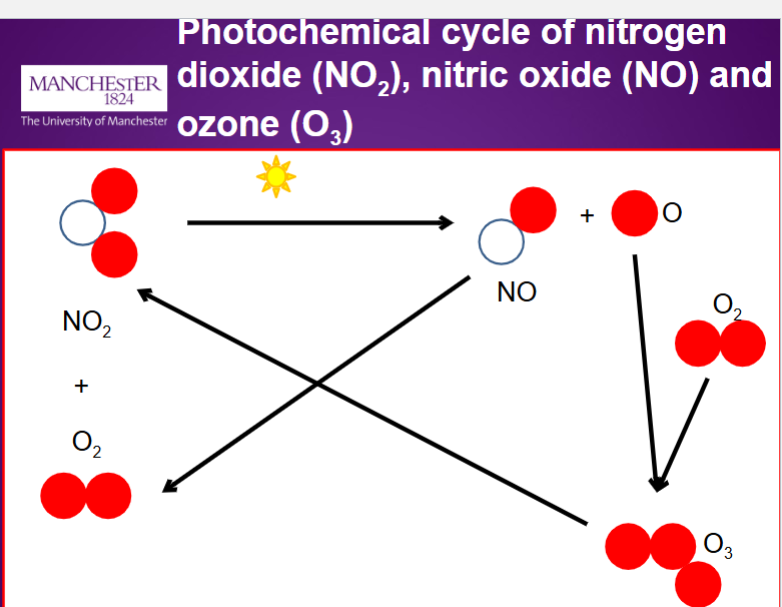
Sunlight photolyses NO₂ into NO + O.
The O atom quickly forms O₃. instantaneously
NO rapidly destroys O₃, regenerating NO₂.
Recap of cycle:
What are is the most common reaction in atmosphere?
Simple bimolecular reactions that are first order in each reactant, so overall second order.